Abstract
Adenosine 3',5'-cyclic monophosphate (cAMP) participates in the regulation of numerous cellular functions, including the Na+-K+-ATPase (sodium pump). Ouabain, used in the treatment of several heart diseases, is known to increase cAMP levels but its effects on the atrium are not understood. The aim of the present study was to examine the effect of ouabain on the regulation of atrial cAMP production and its roles in atrial endothelin-1 (ET-1) secretion in isolated perfused beating rabbit atria. Our results showed that ouabain (3.0 µmol/L) significantly increased atrial dynamics and cAMP levels during recovery period. The ouabain-increased atrial dynamics was blocked by KB-R7943 (3.0 µmol/L), an inhibitor for reverse mode of Na+-Ca2+ exchangers (NCX), but did not by L-type Ca2+ channel blocker nifedipine (1.0 µmol/L) or protein kinase A (PKA) selective inhibitor H-89 (3.0 µmol/L). Ouabain also enhanced atrial intracellular cAMP production in response to forskolin and theophyline (100.0 µmol/L), an inhibitor of phosphodiesterase, potentiated the ouabain-induced increase in cAMP. Ouabain and 8-Bromo-cAMP (0.5 µmol/L) markedly increased atrial ET-1 secretion, which was blocked by H-89 and by PD98059 (30 µmol/L), an inhibitor of extracellular-signal-regulated kinase (ERK) without changing ouabain-induced atrial dynamics. Our results demonstrated that ouabain increases atrial cAMP levels and promotes atrial ET-1 secretion via the mitogen-activated protein kinase (MAPK)/ERK signaling pathway. These findings may explain the development of cardiac hypertrophy in response to digitalis-like compounds.
Go to : 
The Na+-K+-ATPase (sodium pump) is present in the membranes of all cells. It generates Na+ and K+ ion gradients that serve in the secondary co- and counter-transport processes that maintain gradients of H+, Ca2+, Cl-, and other organic molecules. The accumulation of intracellular Na+ in turn activates the Na+-Ca2+ exchanger (NCX) [12] and L-type Ca2+ channels [3], which have wide-ranging effects on the intracellular milieu and, hence, on cell function, including excitability, energy metabolism, and excitation-contraction coupling [4]. The Na+/K+-ATPase also regulates the growth and phenotype of cardiac myocytes. Thus, alterations in this sodium pump, whether drug-induced, by the actions of endogenous digitalis-like compounds, or through pathological down-regulation, are likely to be involved in the development of cardiac hypertrophy and failure [5].
The activity of the Na+-K+-ATPase is modulated by adenosine 3',5'-cyclic monophosphate (cAMP), a second messenger that participates in the regulation of numerous cell functions [6]. cAMP is synthesized by adenylate cyclase (AC) and degraded by phosphodiesterases (PDEs). It is required for the activation of protein kinase A (PKA). The PKA signaling pathway is one of the most important cellular signaling pathways but it has also been shown to promote mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) phosphorylation in the heart during morphine withdrawal [7].
Ouabain and other cardiotonic steroids bind to the cardiac Na+-K+-ATPase and inhibit its activity [8910]. Ouabain has also been shown to increase cAMP levels in rat optic nerve astrocytes [11], dog renal cortex [12], rat brain [13], and cultured renal papillary-collecting tubule cells [14]. However, the effect of ouabain on the regulation of cAMP production in the atrium and its roles in atrial function are not well understood. In our previous study we have observed that ouabain promotes atrial natriuretic peptide (ANP) secretion via endothelin-1 (ET-1)-ETB receptor-mediated pathway [8].
The aims of the present study was to examine the effect of ouabain on the regulation of atrial cAMP production and its roles on atrial ET-1 secretion in isolated perfused beating rabbit atria.
Go to : 
The atria of New Zealand white rabbits of either sex were removed and weighed (mean wet weight=0.25±0.08 g). Isolated perfused beating left atria were prepared using previously described methods [15]. Transmural electrical field stimulation of 1.5 Hz (0.3 ms, 30~40 V) was applied to the perfused atria using a luminal electrode. During stimulation, the atria were perfused with HEPES buffer using a peristaltic pump (1 ml/min) that allowed atrial pacing for measurements of the changes in atrial volume (stroke volume), cAMP efflux, and ET-1 secretion. The HEPES buffer contained (in mmol/L) 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 10.0 glucose, 10.0 HEPES (pH 7.4 with NaOH) and 0.1% bovine serum albumin.
Left atria were obtained from adult New Zealand white rabbits. The atrial tissue was placed in 2 ml of ice-cold phosphate buffer (30 mM, pH 7.2) containing 120 mM NaCl and 1 mM phenanthroline, finely minced, and homogenized at 4℃ by three 30-s bursts of maximal speed using a Tissue Tearor (Biospec, Racine, WI, USA). The homogenate was centrifuged at 1,000 g for 10 min at 4℃. The supernatant was then centrifuged at 40,000 g for 60 min at 4℃. The pellet, containing the cell membranes, was washed three times with Tris-HCl (50 mM, pH 7.4) containing 1 mM EDTA. The membranes were suspended with sonication in the same buffer. Protein content was determined by a bicinchoninic acid assay kit (Sigma Chemical, St. Louis, MO, USA).
The atrium was perfused for 60 min to stabilize the parameters for ET-1 secretion and cAMP efflux as well as atrial dynamics. The perfusates were collected at 2-min intervals at 4℃ for measurements of ET-1 and cAMP levels. The cycle lasted 12 min.
The control cycle (12 min) was followed by the infusion of 3.0 µmol ouabain/L for one cycle and then seven recovery cycles, during which HEPES buffer was infused. The cAMP levels in the perfusates were measured by radioimmunoassay.
The roles of PKA, L-type Ca2+ channels, and the NCX on the regulation of ouabain-induced atrial mechanical action were examined as follows: After one control period, one cycle with the treatment agent was followed by one cycle of infusion of the treatment agents plus ouabain (3.0 µmol/L) and then by two cycles of recovery. The treatment agents were as follows: 1) H-89 (3.0 µmol/L), a selective PKA inhibitor; 2) nifedipine (1.0 µmol/L), an L-type Ca2+ channel inhibitor; and 3) KB-R7943 (1.0 µmol/L), an inhibitor of the reverse mode of NCX.
The mechanism by which ouabain regulates cAMP content was investigated in atrial myocyte membranes. The atrial membranes were incubated, or not (control), with ouabain (3.0 µmol/L), forskolin (0.1 µmol/L), ouabain+forskolin, the inhibitor of PDE theophyline (100.0 µmol/L), theophyline +ouabain (n=6 membrane preparations per group). Incubations were carried out for 30 min with shaking. The cAMP content was measured by radioimmunoassay (RIA).
The effect of ouabain-induced cAMP levels on the regulation of ET-1 secretion was examined in perfused beating rabbit atria. After one control period, one cycle with the treatment agent was followed by one cycle of H-89 (3.0 µmol/L) infusion, one cycle of H-89 plus ouabain (3.0 µmol/L), and then two cycles of recovery in the presence of H-89. Next, one 12-min control cycle was followed by 2×12-min cycles of 8-bromo-cAMP (0.5 µmol/L). To investigate the effect of cAMP on the regulation of ET-1 secretion, after one control cycle, two cycles of H-89 or the ERK inhibitor PD98059 (30 µmol/L) were followed by two cycles of H-89 plus 8-bromo-cAMP or PD98059 plus 8-bromo-cAMP.
The level of immunoreactive cAMP in the perfusates was measured by a specific RIA as described previously [15]. The standard and the sample were placed in polyethylene tubes containing 60 µl of acetate buffer (50.0 mmol/L, pH 4.8) together with cAMP antibody and tracer. The reaction was stopped by the addition of activated carbon. The samples were centrifuged at 3,000 rpm for 24 h at 4℃ and the radiation in the sediment was measured to determine the cAMP concentration.
The level of immunoreactive ET-1 in the perfusates was measured in a competitive RIA (North Institute of Biological Technology, Beijing, China) as described previously [16]. Prior to the assay the samples were extracted using Sep-Pak C18 cartridges (Waters, Milford, MA, USA). Intra-assay and inter-assay coefficients of variation were less than 10 and 15%, respectively. The sensitivity of the assay was 0.5 pg/tube; cross-reactivity with ET-2 and ET-3 was less than 1%. All samples were assayed in the same run and at least in duplicate. The amount of ET-1 in the perfusate is expressed as pg/min/g of wet atrial tissue.
The significance of differences between values was determined by one-way ANOVA followed by Dunnett's multiple comparison and an unpaired t-test. The data are expressed as mean±SEM. Statistical significance was defined at p<0.05.
Go to : 
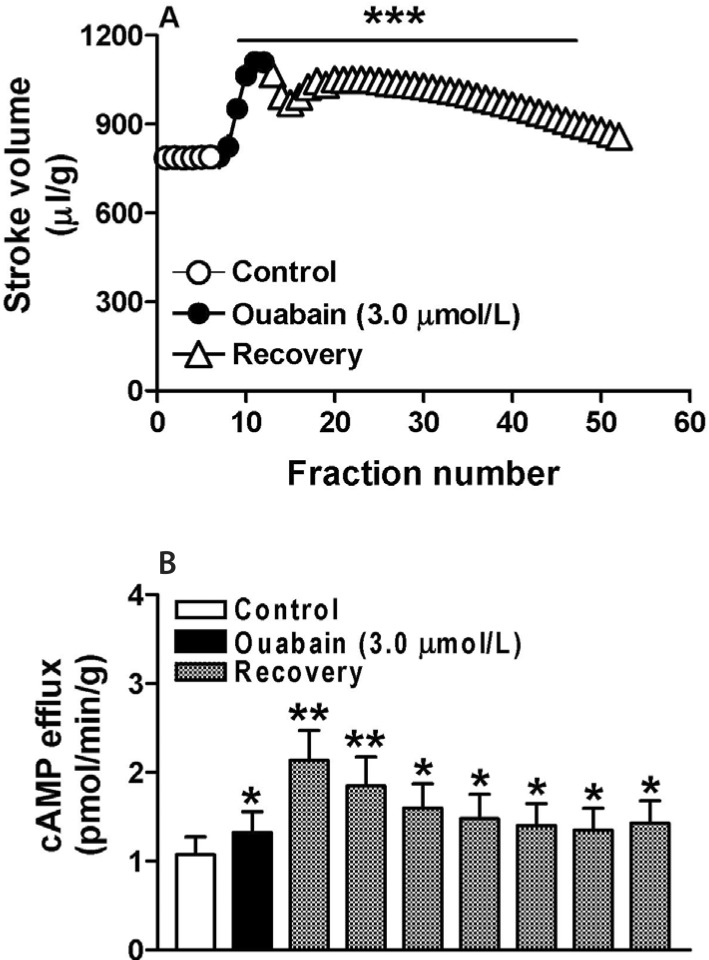
Atrial stroke volume increased at an early point during treatment with ouabain (n=6, p<0.001 vs. control cycle; Fig. 1A) and continued during the recovery period. The atrial cAMP content was also induced by ouabain and showed similar characteristics to the changes in atrial stroke volume during the recovery period (n=6, p<0.05 vs. control cycle; Fig. 1B). These data suggested that in rabbit atria ouabain not only induces positive inotropic action but also promotes cAMP production.
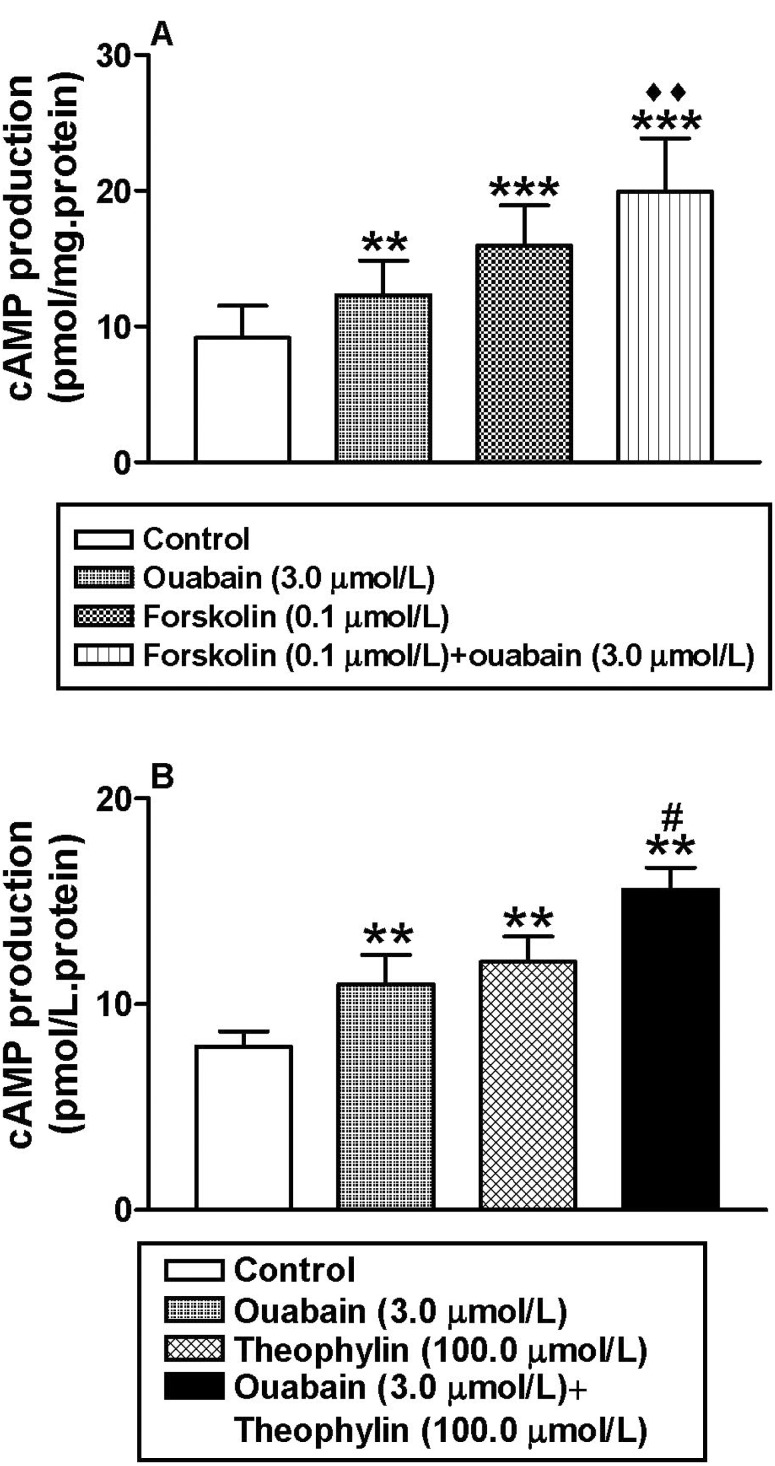
To determine the mechanism by which ouabain increases atrial cAMP content, cardiac atrial myocyte membranes were incubated with ouabain and/or forskolin. Both agents significantly increased atrial cAMP levels (n=6, p<0.01 and p<0.001 vs. control group, respectively; Fig. 2A), and showed a synergistic effect when added together (n=6, p< 0.001 vs. control group, p<0.01 vs. ouabain group; Fig. 2A). In addition, theophyline also significantly increased the atrial cAMP content (n=6, p<0.01 vs. control group, Fig. 2B). In the presence of theophyline, ouabain further increased intracellular cAMP production (n=6, p<0.05 vs. ouabain group; Fig. 2B). These results indicated that ouabain promotes atrial cAMP production by cativation of AC.
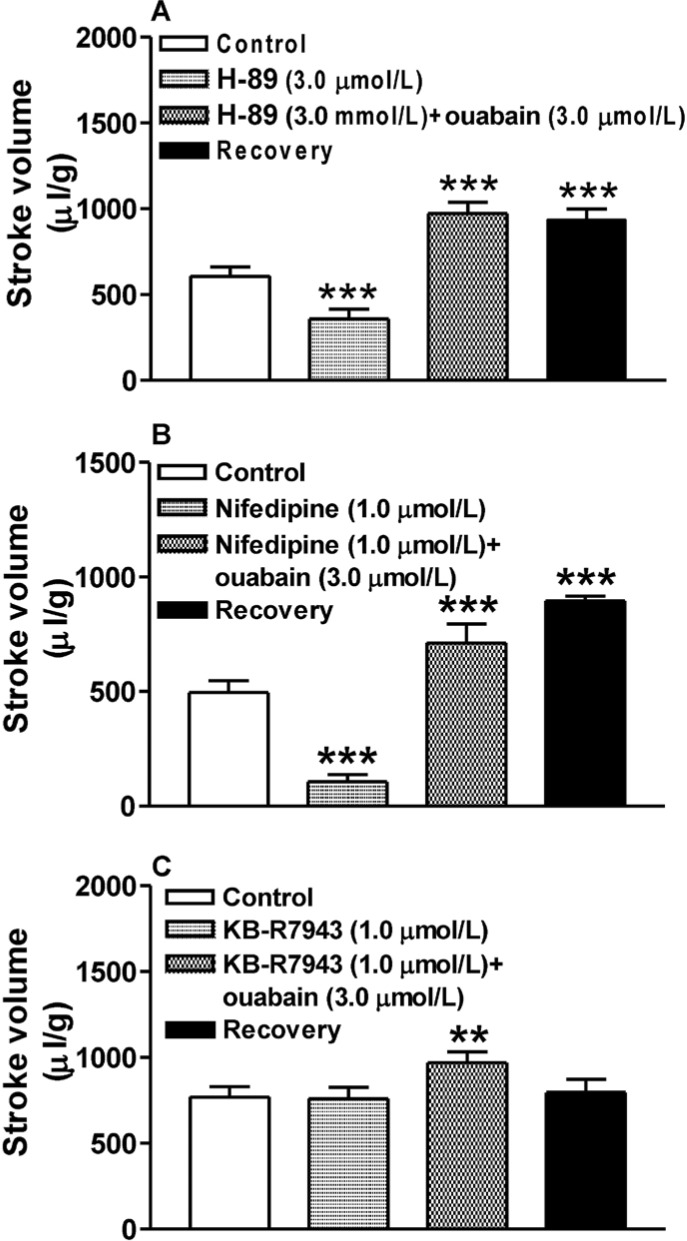
The effect of the ouabain-induced increase in cAMP on atrial dynamics was tested in atria treated with ouabain and inhibitors of PKA, L-type Ca2+ channels, and the NCX. Neither H-89 nor nifedipine modulated the ouabain-induced increase in atrial stroke volume, either during the ouabain treatment period or during the recovery period (n=6, p<0.001 vs. H-89; Fig. 3A, B). However, reverse mode of the NCX inhibitor KB-R7943 completely abolished ouabain induction of atrial stroke volume during the recovery stage (n=6, p>0.05 vs. KB-R7943; Fig. 3C). These results indicated that ouabain increases atrial dynamics mainly by inhibiting the Na+-K+-ATPase whereas the ouabain-induced increase in cAMP has no effect.
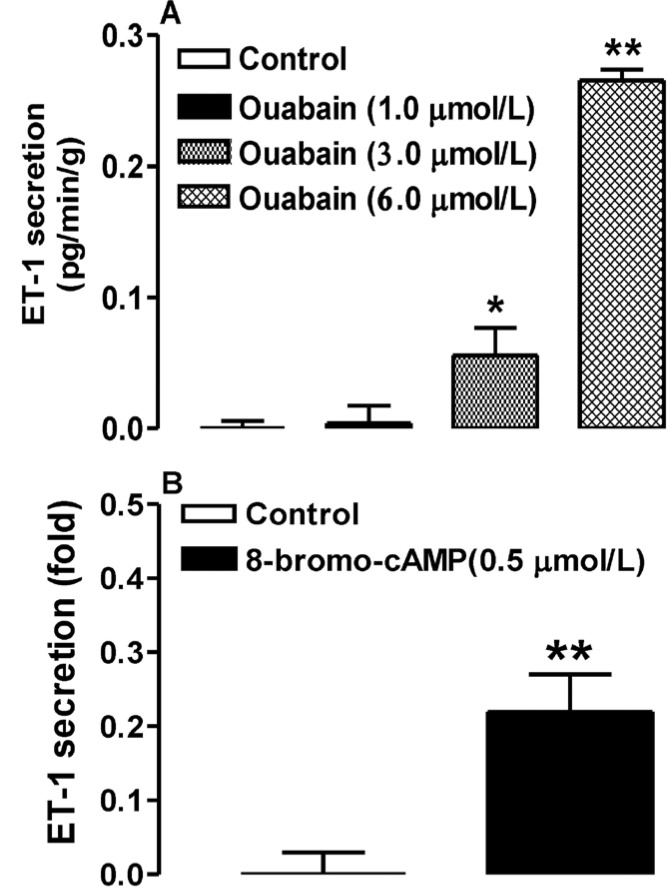
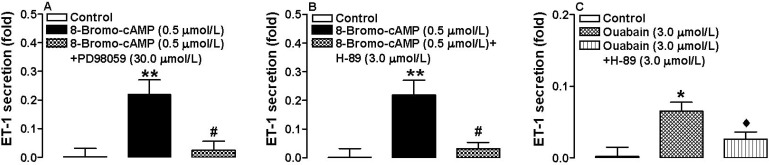
We then asked whether the ouabain-induced increase in cAMP has an effect on atrial ET-1 secretion. Atria were treated with different doses of ouabain (1.0, 3.0, and 6.0 µmol/L) together with membrane permeable 8-bromo-cAMP, PD98059, or H-89. At doses of 3.0 and 6.0 µmol/L, ouabain significantly increased ET-1 secretion (n=6, p< 0.05, p<0.01 vs. control respectively; Fig. 4A), as did 8-bromo-cAMP (n=6, p<0.01 vs. control; Fig. 4B). However, the latter effect was completely blocked by PD98059 and H-89 (n=6, p<0.01 vs. 8-bromo-cAMP; Fig. 5A, B). H-89 also completely abolished the ouabain-induced increase in ET-1 secretion (n=6, p<0.01 vs. 8-bromo-cAMP; Fig. 5C). These findings suggest that the cAMP-dependent PKA and MAPK/ERK signaling pathways are involved in cAMP-induced atrial ET-1 secretion.
Go to : 
In the present study, ouabain increased atrial cAMP levels in isolated perfused beating rabbit atria. The increase in cAMP in turn stimulated atrial ET-1 secretion. Ouabain increased atrial dynamics mainly by inhibiting the Na+-K+-ATPase, and not through the ouabain-induced increase in atrial cAMP production.
The intracellular cAMP content is determined by the rate of cAMP generation by AC and by its degradation by PDEs. The atrial concentration of cAMP was previously shown to correlate positively with its production by atrial myocytes [15]. In the present study, we showed that ouabain significantly increased the efflux of atrial cAMP in a sustained manner, as efflux continued during the recovery period, after ouabain infusion. In addition, ouabain also enhanced intracellular cAMP production with atrial membrane and the enhancement by ouabain of cellular cAMP production was amplified with PED inhibitor theophline. In the presence of the AC activator forskolin, ouabain further elevated atrial cAMP production. These findings are similar to those that ouabain increases intracellular cAMP production in the intestinal mucosa, pancreatic islets, and brain by stimulating AC [131718].
We found that neither the PKA inhibitor H-89 nor the L-type Ca2+ channel inhibitor nifedipine altered ouabain-induced atrial dynamics, whereas the latter effect was completely abolished during the recovery period by the reverse mode of NCX inhibitor KB-R7943. These results suggested that ouabain increases atrial dynamics mainly by inhibiting the Na+-K+-ATPase, independent of the ouabain-induced increase in atrial cAMP. In previous reports, inhibitors of PDE3 and PDE4 increased atrial cAMP production but failed to increase atrial dynamics, most likely because these responses were compartmentalized [19]. Early studies showed that the PDE4 inhibitor rolipram does not exert positive inotropic action in either guinea pig atria [20] or rat ventricular papillary muscle [21]. Other studies also reported that the increase in cAMP production induced by the inhibition of PDE3 and PDE4 was localized, resulting in different effects on the regulation of calcium transients and myocardial mechanical activity [2223]. Our findings are in agreement with those results.
To further investigate the role of ouabain-induced cAMP on rabbit atria, we examined the effects of ouabain and 8-Bromo-cAMP on atrial ET-1 secretion and determined that secretion was significantly increased by both agents and blocked by the PKA inhibitor H-89 and by the MAPK/ERK inhibitor PD98059. cAMP-dependent PKA signaling is related to the MAPK/ERK signaling pathway [7]. In previous work we showed that the MAPK/ERK signaling pathway is involved in the regulation of ouabain-induced atrial ET-1 secretion and to promotes atrial ANP release[8]. According to the results of this study, cAMP induced by ouabain stimulates atrial ET-1 secretion by activating PKA and the MAPK/ERK signaling pathways. These findings may explain the development of cardiac hypertrophy in response to digitalis-like compounds: by the ET-1 induced activation of the MAPK pathway, which is a key signaling route in the mechanicalload-induced hypertrophic process [5].
In conclusion, this study demonstrated that ouabain induces atrial cAMP production by activating AC and is involved in the regulation of atrial ET-1 secretion via PKA and the MAPK/ERK signaling pathways.
Go to : 
Notes
Author contributions: L.P. and P.L. performed atrial perfused experiments. Q.Z., performed endothelin-1 measurement. L.L. and L.H. performed cAMP measurement. B.C. and X.C. designed experiments and wrote the manuscript.
Go to : 
ACKNOWLEDGEMENTS
We thank Shan-ji Jin and Su-xiang Li for expert technical assistance. This work was supported by the National Natural Science Foundation of China (No. 81360061) and the Jilin Provincial Research Foundation for Basic Research, China (No. 20130101133JC).
Go to : 
References
1. Altamirano J, Li Y, DeSantiago J, Piacentino V 3rd, Houser SR, Bers DM. The inotropic effect of cardioactive glycosides in ventricular myocytes requires Na+-Ca2+ exchanger function. J Physiol. 2006; 575:845–854. PMID: 16825310.
2. Satoh H, Ginsburg KS, Qing K, Terada H, Hayashi H, Bers DM. KB-R7943 block of Ca2+ influx via Na+/Ca2+ exchange does not alter twitches or glycoside inotropy but prevents Ca2+ overload in rat ventricular myocytes. Circulation. 2000; 101:1441–1446. PMID: 10736290.
3. Saini HK, Dhalla NS. Sarcolemmal cation channels and exchangers modify the increase in intracellular calcium in cardiomyocytes on inhibiting Na+-K+-ATPase. Am J Physiol Heart Circ Physiol. 2007; 293:H169–H181. PMID: 17322410.
4. White CN, Liu CC, Garcia A, Hamilton EJ, Chia KK, Figtree GA, Rasmussen HH. Activation of cAMP-dependent signaling induces oxidative modification of the cardiac Na+-K+ pump and inhibits its activity. J Biol Chem. 2010; 285:13712–13720. PMID: 20194511.
5. Xie Z, Askari A. Na+/K+-ATPase as a signal transducer. Eur J Biochem. 2002; 269:2434–2439. PMID: 12027880.
6. Butcher RW, Sutherland EW. The effects of the catecholamines, adrenergic blocking agents, prostaglandin E1, and insulin on cyclie AMP levels in the rat epididymal fat pad in vitro. Ann N Y Acad Sci. 1967; 139:849–859. PMID: 4382732.
7. Almela P, Atucha NM, Milanés MV, Laorden ML. Cross-talk between protein kinase A and mitogen-activated protein kinases signalling in the adaptive changes observed during morphine withdrawal in the heart. J Pharmacol Exp Ther. 2009; 330:771–782. PMID: 19567779.

8. Liu LP, Hong L, Yu L, Li HY, Ding DZ, Jin SJ, Cui X. Ouabain stimulates atrial natriuretic peptide secretion via the endothelin-1/ET(B) receptor-mediated pathway in beating rabbit atria. Life Sci. 2012; 90:793–798. PMID: 22521291.

9. Baudouin-Legros M, Brouillard F, Tondelier D, Hinzpeter A, Edelman A. Effect of ouabain on CFTR gene expression in human Calu-3 cells. Am J Physiol Cell Physiol. 2003; 284:C620–C626. PMID: 12556359.

10. Bagrov AY, Fedorova OV, Austin-Lane JL, Dmitrieva RI, Anderson DE. Endogenous marinobufagenin-like immunoreactive factor and Na+, K+ ATPase inhibition during voluntary hypoventilation. Hypertension. 1995; 26:781–788. PMID: 7591018.
11. Mandal A, Delamere NA, Shahidullah M. Ouabain-induced stimulation of sodium-hydrogen exchange in rat optic nerve astrocytes. Am J Physiol Cell Physiol. 2008; 295:C100–C110. PMID: 18448627.

12. Westenfelder C, Sack E, Stuart E, Earnest W, Baranowski R, Kurtzman N. Effect of ouabain on basal and PTH-stimulated cAMP production in dog renal cortex (Abstract). Kidney Int. 1979; 16:844.
13. Mørk A, Geisler A. A comparative study on the effects of tetracyclines and lithium on the cyclic AMP second messenger system in rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 1995; 19:157–169. PMID: 7708928.

14. Ishikawa S, Saito T. Effect of ouabain on cellular free calcium and cellular cyclic AMP production in response to arginine vasopressin in rat renal papillary collecting tubule cells in culture. J Endocrinol. 1989; 121:467–477. PMID: 2547011.

15. Cui X, Lee SJ, Kim SZ, Kim SH, Cho KW. Effects of pituitary adenylate cyclase activating polypeptide27 on cyclic AMP efflux and atrial dynamics in perfused beating atria. Eur J Pharmacol. 2000; 402:129–137. PMID: 10940366.

16. Cho KW, Kim SH, Hwang YH, Seul KH. Extracellular fluid translocation in perfused rabbit atria: implication in control of atrial natriuretic peptide secretion. J Physiol. 1993; 468:591–607. PMID: 8254526.

17. Bakker R, Groot JA. cAMP-mediated effects of ouabain and theophylline on paracellular ion selectivity. Am J Physiol. 1984; 246:G213–G217. PMID: 6320677.

18. Gagerman E, Hellman B, Täljedal IB. Effects of ouabain on insulin release, adenosine 3',5'-monophosphate and guanine 3',5'-monophosphate in pancreatic islets. Endocrinology. 1979; 104:1000–1002. PMID: 86435.

19. Cui X, Wen JF, Jin H, Li D, Jin JY, Kim SH, Kim SZ, Lee HS, Cho KW. Subtype-specific roles of cAMP phosphodiesterases in regulation of atrial natriuretic peptide release. Eur J Pharmacol. 2002; 451:295–302. PMID: 12242091.

20. Muller B, Lugnier C, Stoclet JC. Involvement of rolipram-sensitive cyclic AMP phosphodiesterase in the regulation of cardiac contraction. J Cardiovasc Pharmacol. 1990; 16:796–803. PMID: 1703603.

21. Shahid M, Nicholson CD. Comparison of cyclic nucleotide phosphodiesterase isoenzymes in rat and rabbit ventricular myocardium: positive inotropic and phosphodiesterase inhibitory effects of Org 30029, milrinone and rolipram. Naunyn Schmiedebergs Arch Pharmacol. 1990; 342:698–705. PMID: 1710786.

22. Bers DM, Ziolo MT. When is cAMP not cAMP? Effects of compartmentalization. Circ Res. 2001; 89:373–375. PMID: 11532895.
23. Hohl CM, Li QA. Compartmentation of cAMP in adult canine ventricular myocytes. Relation to single-cell free Ca2+ transients. Circ Res. 1991; 69:1369–1379. PMID: 1682066.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download