INTRODUCTION
METHODS
Cell culture and transfection
Electrophysiology
Solutions and drugs
Data analysis
RESULTS
Concentration-dependent and reversible inhibition of Kv1.5 by paroxetine
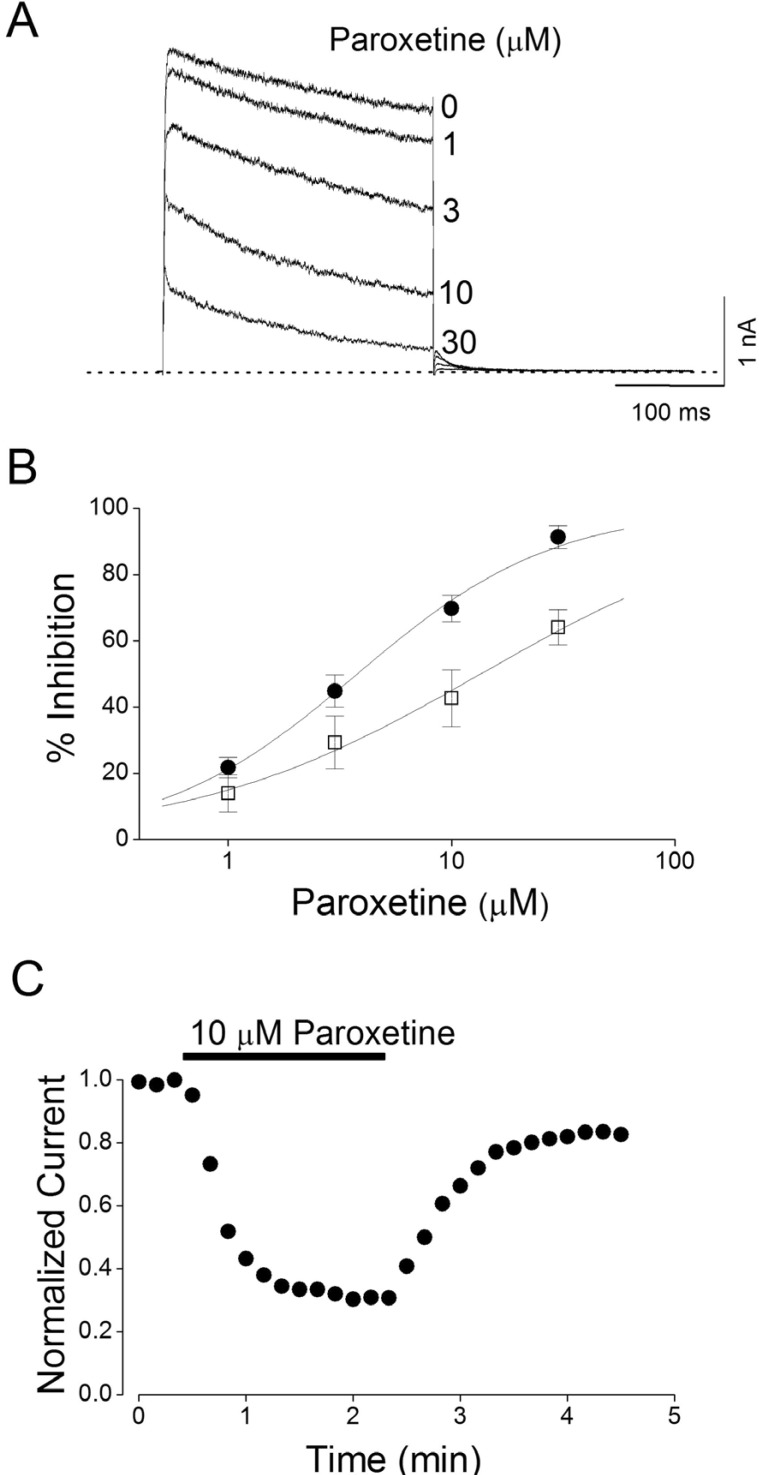 | Fig. 2Concentration-dependent and reversible inhibition of Kv1.5 by paroxetine.(A) Superimposed current traces were produced by applying 250-ms depolarizing pulses from a holding potential of -80 to +50 mV followed by a 250-ms repolarizing pulse to -40 mV every 10 s in the absence and presence of 1, 3, 10, and 30 µM paroxetine, as indicated. The dotted line represents zero current. (B) Concentration-dependent curve of inhibition by paroxetine. Current amplitudes of Kv1.5 measured at the end of the depolarizing pulses (closed circle) and peak current (open square) were used and the respective percentage inhibitions were plotted against various concentrations of paroxetine. The solid line is fitted to the data points by the Hill equation. Data are expressed as mean±S.E.M. (C) Representative time course for inhibition in the presence of 10 µM paroxetine. The current amplitudes were measured at the end of a 250-ms depolarizing pulses from a holding potential of -80 to +50 mV every 10 s in the presence of 10 mM paroxetine and normalized to the first current amplitude and the normalized data were plotted as a function of time.
|
Voltage-dependent inhibition of Kv1.5 by paroxetine
 | Fig. 3Voltage dependence of paroxetine-induced inhibition of Kv1.5 currents.The Kv1.5 currents were produced by applying 250-ms pulses between -60 and +50 mV in 10-mV increments followed by a 250-ms repolarizing pulse to -40 mV every 10 s, from a holding potential of -80 mV under control conditions (A), and after the addition of 10 µM paroxetine (B). The dotted lines in (A) and (B) represent zero current. (C) Resultant I~V relationships taken at the end of the test pulses in the absence (open circle) and presence (closed circle) of 10 µM paroxetine. (D) Percentage current inhibition (closed square) from data in (C) was plotted against the membrane potential. For potentials positive to 0 mV, the data of percentage current inhibition was recalculated by using ln{(IControl-IParoxetine)/IParoxetine} (closed triangle) and replotted against membrane potential. The voltage dependence was linear fitted with equation 7 (see MATERIALS AND METHODS), shown by the solid line with the indicated values for the equivalent electrical distance (δ=0.32±0.07, n=5). The dotted line represents the activation curve of Kv1.5 under control conditions, which was obtained from a deactivating tail current amplitude at -40 mV after 250-ms depolarizing pulses to potentials between -60 to +50 mV in steps of 10 mV from a holding potentials of -80 mV and thereafter normalization using equation 2 (see MATERIALS AND METHODS).
|
Time-dependent inhibition of Kv1.5 by paroxetine
 | Fig. 4Concentration-dependent kinetics of Kv1.5 inhibition by paroxetine.(A) Superimposed Kv1.5 current traces were elicited by applying 250-ms depolarizing pulses from a holding potential of -80 to +50 mV every 10 s in the presence of paroxetine (1, 3, 10, and 30 µM). The dotted line represents zero current. (B) The drug-induced time constants (τD) were obtained by a selection of the fast time constant from a double exponential fitting to the decaying traces of Kv1.5 currents. The inverse of τD obtained at +50 mV was plotted versus paroxetine concentrations. The solid line represents the least-squares fit of the data to the relation 1/τD=k+1[D]+k-1. A binding rate constant (k+1) and an unbinding rate constant (k-1) were obtained from the slope and intercept values of the fitted line. Data are expressed as mean±S.E.M.
|
 | Fig. 5Effects of paroxetine on deactivation kinetics of Kv1.5.(A) Tail currents were induced at a 250-ms repolarizing pulses of -40 mV after a 250-ms depolarizing pulse of +50 mV from a holding potential of -80 mV in the absence and presence of 10 µM paroxetine. The dotted lines represent a zero current. Tail crossover phenomenon (indicated by the arrow) observed by superimposing the two tail currents. (B) Deactivation time constants (τ) obtained from (A). The deactivation time constants (τ) were obtained from a single exponential fitting to the decaying traces of Kv1.5 currents. The symbol * indicates a statistically significant difference (n=4, p<0.05 versus control data). Data are expressed as mean±S.E.M.
|
Use- and frequency-dependent inhibition of Kv1.5 by paroxetine
 | Fig. 6Effects of repetitive depolarization on paroxetine-induced inhibition of Kv1.5 currents.(A) Original current traces under control conditions and in the presence of 10 µM paroxetine obtained by applying 20 repetitive 125-ms depolarizing pulses of +50 mV from a holding potential of -80 mV at 1 and 2 Hz. The dotted lines represent a zero current. (B) Plot of normalized current at 1 and 2 Hz under control conditions (open circle and open triangle, n=4) and in the presence of 10 µM paroxetine (closed circle and closed triangle, n=4) as a function of the number of pulses. Peak amplitudes of the current from every pulse were normalized to the peak amplitudes of current obtained from the first pulse. (C) Relative current (IParoxetine/IControl) plotted at 1 (open square) and 2 Hz (closed square) from (B) as a function of the number of pulses. Data are expressed as mean±S.E.M.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download