INTRODUCTION
METHODS
Tissue preparation
Preparation of isolated single cells
Voltage-clamp patch experiments
Immunohistochemical labeling of TASK-2 channels
Western blot
Solution and drugs
Statistics
RESULTS
Characterization of the isometric contractions of murine uterus longitudinal muscle
 | Fig. 1Effect of quinidine on the isometric contraction of uterine longitudinal muscle in mice.(A) Oxytocin (OXT) produced initial and tonic contractions; however, tonic contractions were not sustained in non-pregnant myometrium (NP, non-pregnant; TP, term-pregnant). (B) Quinidine (10, 20, and 30 µM) produced concentration-dependent phasic contractions. Tonic contractions were also produced at high concentrations of quinidine in pregnant myometrium. (C) Quinidine was applied to myometrium in the presence of TEA (10 mM). TEA (10 mM) produced strong phasic contractions in non-pregnant myometrium (1.2±0.15 g) but not in pregnant myometrium. Quinidine (10~30 µM), even in the presence of TEA, increased contraction frequency in non-pregnant myometrium and produced stronger contractions in pregnant myometrium.
|
Effect of quinidine on isometric contraction of murine uterus longitudinal muscle
Regulation of murine pregnant longitudinal myometrial contractility by TASK-2 channel inhibitors
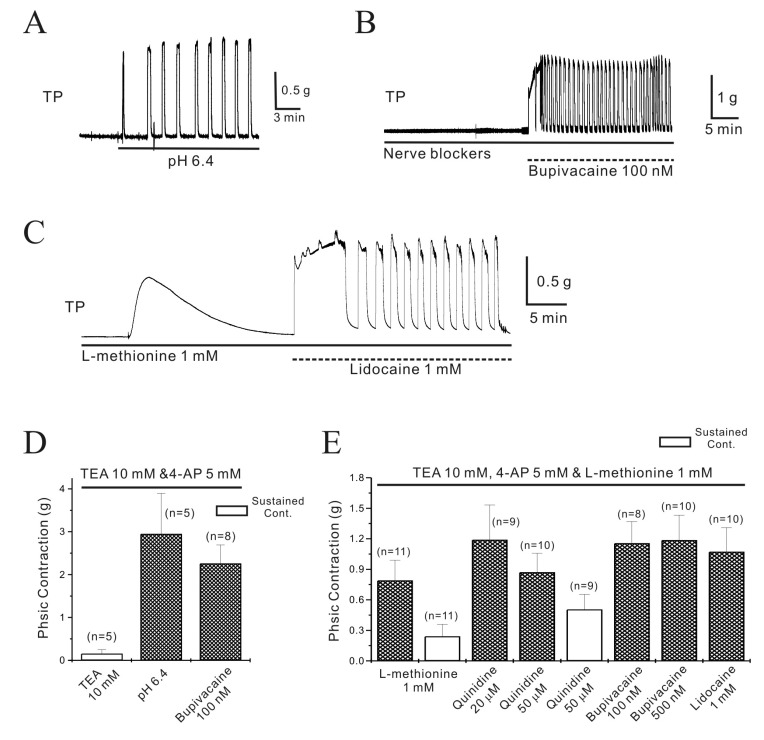 | Fig. 2Regulation of pregnant mouse myometrial contractility by TASK-2 channel inhibitors in the presence of TEA and 4-AP.The TASK-2 inhibitors produced contractions in pregnant myometrium in the presence of TEA and 4-AP. (A) Extracellular acidosiso (pHo=6.4) produced contractions in pregnant myometrium. (B) Bupivacaine (100 nM) also produced robust contractions even in the presence of nerve blockers. (C) L-methionine (1 mM), which inhibits stretch-dependent K2P channels (TREK-1), produced contractions that spontaneously decayed to near baseline values within 10~15 min. However, lidocaine produced robust contraction in the presence of L-methionine. (D~E) Data are summarized.
|
Identification of TASK-2 channel current and its regulation in murine non-pregnant and pregnant single myometrial cells
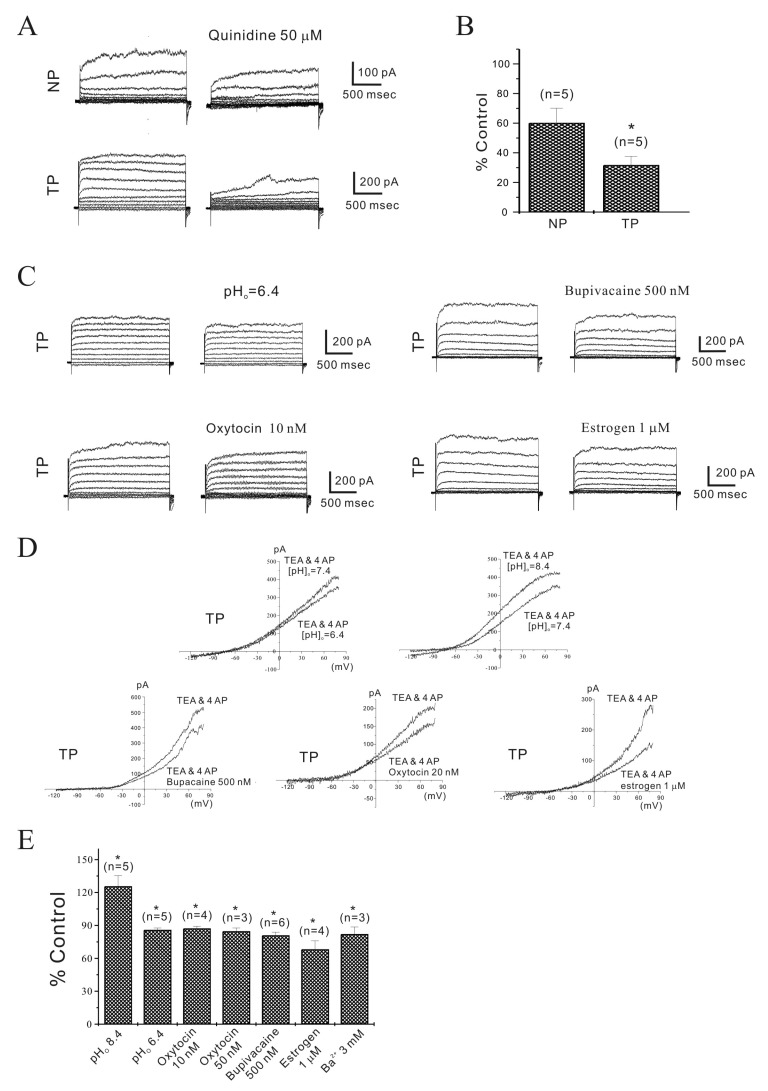 | Fig. 3Regulation of TEA and 4-AP insensitive non-inactivating outward current (NIOK) in single mouse myometrial cells.(A, B) In the presence of TEA (10 mM) and 4-AP (5 mM), NIOK were observed under whole-cell voltage clamp. Quinidine (50 µM) inhibited NIOK currents more significantly in pregnant cells. (C) NIOK was inhibited by pHo=6.4, bupivacaine, oxytocin (OXT), and estrogen in pregnant myometrial cells. (D) Representative I/V relationships are shown. (E) Data are summarized. NIOK was inhibited to 126, 86, 87, 85, 81, 68 and 78% of the control by pHo=8.4, pHo=6.4, OXT (10 and 50 nM), bupivacaine (500 nM), estrogen (1 µM), and Ba2+ (3 mM), respectively (n= 5, 5, 4, 3, 6, 4 and 3, respectively; *p<0.05). Such as TASK-2 is very promising for managing labor at term and developing target medicines. As shown in Fig. 3C~3E, OXT (10 and 50 nM) inhibited the NIOK current to 87±1.94% and 85±2.93% in pregnant cells in a reversible manner, respectively (p<0.05). Estrogen (1 µM) also inhibited the NIOK current to 68±7.65% (n=4; p<0.05).
|
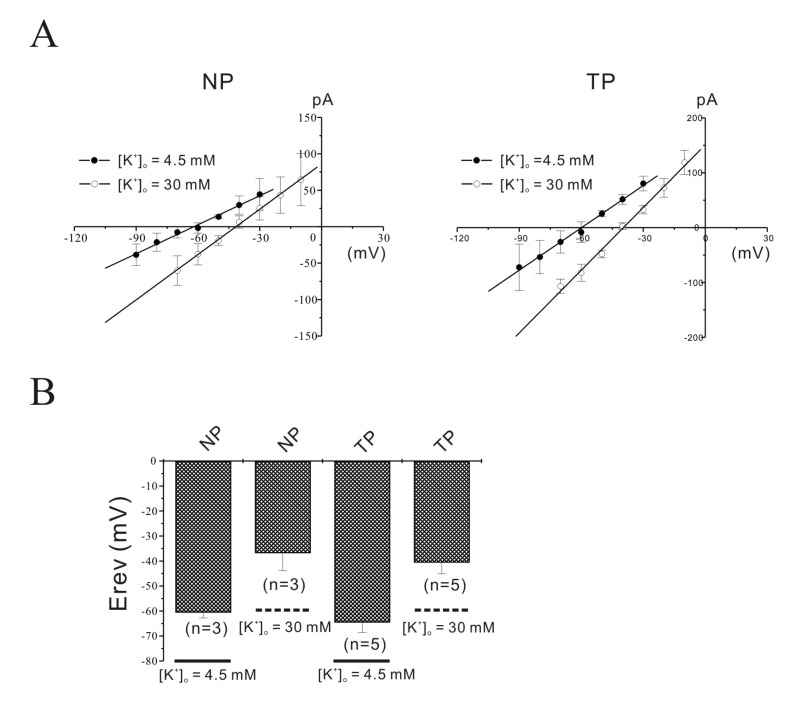 | Fig. 4Identification of NIOK by reversal potentials (Erev).The reversal potentials (Erev) of NIOK were evaluated by changing the external K+ concentration from 4.5 to 30 mM. Erev shifted to new expected values at 30 mM of external K+. Instantaneous tail currents in the NIOK of non-pregnant and pregnant myometrial cells were reversed at –37 mV (n=3) and –41 mV (n=5) by 30 mM K+.
|
Identification of TASK-2 by immunohistochemistry and Western blot in murine myometrium
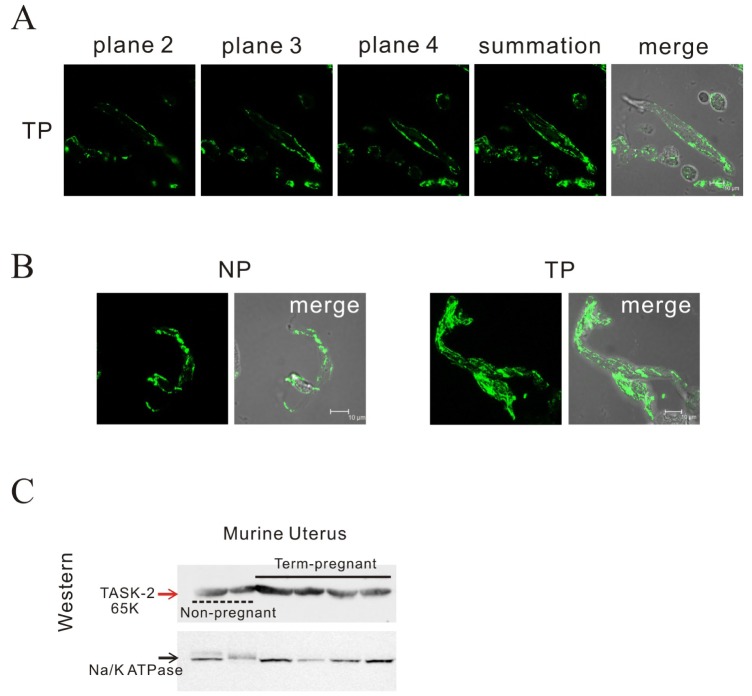 | Fig. 5Identification of TASK-2 by immunohistochemistry and Western blot in mouse myometrium.(A, B) Laser scanning confocal micrograph shows TASK-2 immunoreactivity on the membrane of a single pregnant cell. Confocal images at 2 µm intervals showed localization of the TASK-2 channel on the cell membrane of pregnant myometrial cells. Highly increased immunoreactivity for the TASK-2 antibody in single pregnant myometrial cells ws observed compared to that in non-pregnant cells. (C) TASK-2 was increased in pregnancy compared to the control under Western blot (595 vs 100, p<0.05; n=3 and 4).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download