INTRODUCTION
METHODS
Cell culture
Western blot analysis
Animals
Nitrite and nitrate measurements
DAF-FM DA staining
Arginase activity assay
Vascular reactivity
Statistical analysis
RESULTS
NM stimulates p-eNOS and p-Akt expression in HUVECs
 | Fig. 1Nafamostat mesilate (NM) dose- and time-dependently stimulates phosphorylation of endothelial nitric oxide synthase (eNOS) and Akt in human umbilical vein endothelial cells (HUVECs).(A) Dose dependent effect of NM on eNOS and Akt phosphorylation in HUVECs. Cells were treated with various concentrations (10~1000 ng/ml) of NM for 2 h then harvested for western blot analysis of p-eNOS and p-Akt. The levels of p-eNOS and p-Akt were quantified by densitometric analyses (B and C). (D) Time dependent effect of NM on eNOS and Akt phosphorylation in HUVECs. Cells were treated with 300 ng/mL of NM for various time points starting from 5 min up to 2 h followed by harvesting the cells for western blot analysis. Total forms of the proteins were included as a loading control. The levels of p-eNOS and p-Akt were quantified by densitometric analyses (E and F). All western blots are representative of three independent experiments. The data are presented as the means±SEM of three independent experiments. *p<0.05 compared with control cells.
|
NM stimulates the production of NO in HUVECs
 | Fig. 2Nafamostat mesilate (NM) stimulates the production of nitric oxide and inhibits arginase activity in human umbilical vein endothelial cells (HUVECs).(A) NO production in HUVECs treated with various concentrations (10~1000 ng/ml) of NM for 2 h. (B) Arginase activity measured in HUVECs treated with various concentrations (30~1000 ng/mL) of NM for 2 h. Bars represent means±standard error (n=3).
|
NM stimulates p-eNOS and p-Akt expression ex vivo
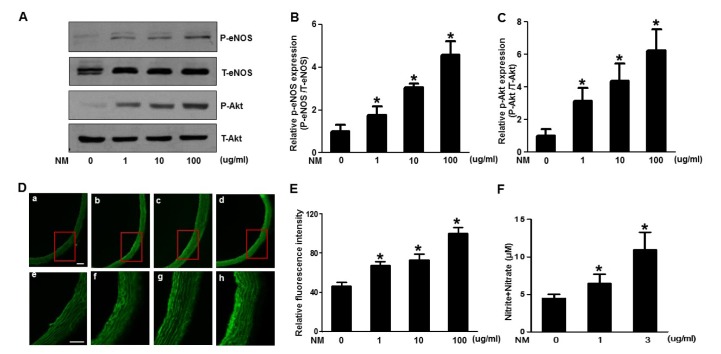 | Fig. 3Nafamostat mesilate (NM) stimulates phosphorylation of Akt/endothelial nitric oxide synthase (eNOS) and production of nitric oxide (NO) ex vivo.(A) Aorta segments were treated with various concentrations (1~100 µg/ml) of NM for 2 h then harvested for western blot analysis of p-eNOS and p-Akt. Total forms of the proteins were included as a loading control. The levels of p-eNOS and p-Akt were quantified by densitometric analyses (B and C). All western blots are representative of three independent experiments. The data are presented as the means±SEM of three independent experiments. *p<0.05 compared with the control. (D) Aorta segments were treated with various concentrations (1~100 µg/ml) of NM for 2 h, stained with DAF dye, and analyzed by microscopy. Images (a, b, c, d) were captured using 100 × magnification. Images (e, f, g, h) were captured using 200 × magnification. Scale bar=40 µm. Corresponding graphs for the relative fluorescence intensity are represented in (E). (F) Rats were treated intravenously with various concentrations (1~3 µg/ml) of NM for 2 h then measured NO production in the plasma. Bars represent means±standard error (n=3).
|
NM stimulates the production of nitric oxide ex vivo
NM improves impaired endothelial-dependent vascular relaxation ex vivo
 | Fig. 4Nafamostat mesilate (NM) improves impaired endothelial-dependent vascular relaxation ex vivo.(A) Aortic rings incubated with various concentrations (10~30 µg/ml) of NM were used to measure vascular reactivity. Increasing aortic relaxation induced by increasing dose of NM in aortic rings preconstricted with phenylephrine (10–5 M) compared with NM plus L-NAME treated aortic rings. *p<0.05compared with the control. (B) Increased bioavailable NO with NM treatment compared to saline control. Bars represent means±standard error (n=3). (C) Graphical representation of NM activity and pathway.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download