INTRODUCTION
METHODS
Animals
In vitro expansion of mouse adipose tissue-derived MSCs
Induction of adipogenic differentiation
Induction of osteogenic differentiation
Induction of chondrocyte differentiation
Ex vivo adipogenic differentiation assay
Quantitative reverse transcription-polymerase chain reaction
Primer sequences
Western blotting
Immunoprecipitation
Retroviral infection of Akt1
Statistical analysis
RESULTS
Lnk promotes the adipogenic differentiation of MSCs
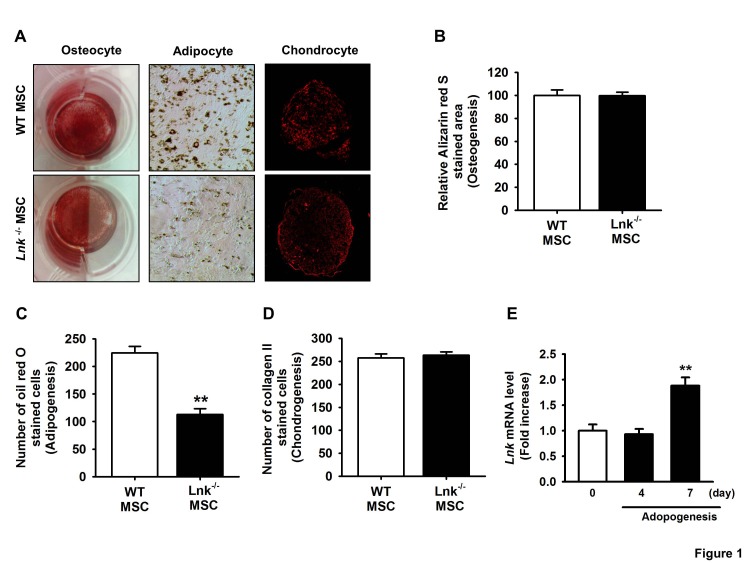 | Fig. 1Differentiation of adipose tissue-derived mesenchymal stem cells.(A) Wild-type (WT) and Lnk–/– mesenchymal stem cells (MSCs) were differentiated in vitro into chondrocytes, osteocytes, and adipocytes. (B) Osteocyte differentiation was quantified as the relative Alizarin red S stained area. Values represent the mean±standard error of mean (SEM). (C) Adipocyte differentiation was quantified as the number of oil red O stained cells. Values represent the mean±SEM; **p<0.01 vs. WT MSC. (D) Chondrocyte differentiation was quantified as the number of collagen II stained cells. Values represent the mean±SEM. (E) Lnk mRNA expression during adipocyte differentiation in wild-type MSCs was measured by performing quantitative reverse transcription-polymerase chain reaction (RT-PCR). Values are expressed as mean±SEM; **p<0.01 vs. values obtained after 4 days.
|
Lnk is required for ex vivo adipogenic differentiation
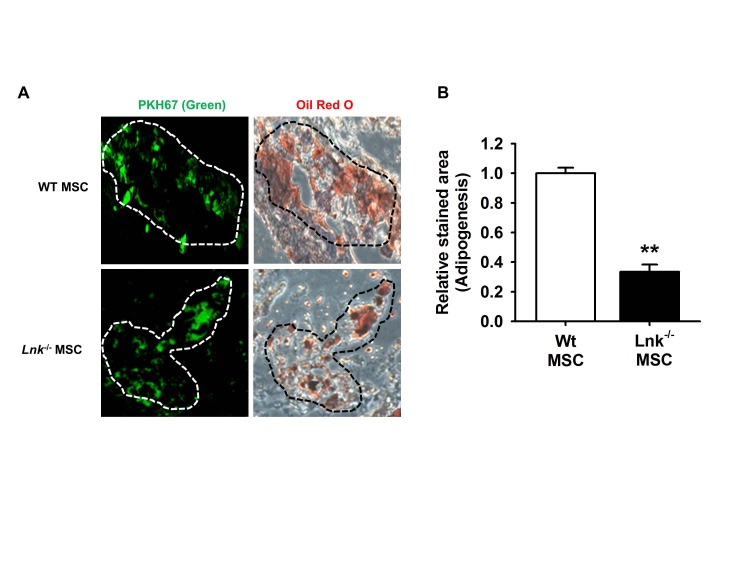 | Fig. 2In vivo adipogenic differentiation of adipose tissue-derived MSCs.(A) Wild-type (WT) or Lnk–/– MSCs were labeled with PKH-67 (green fluorescence), injected subcutaneously into nude mice, and allowed to differentiate for 10 days. Tissue sections showing green fluorescence were stained with hematoxylin or oil red O. Dotted lines indicate PKH-67- or oil red O-stained sites. (B) Adipocyte differentiation was quantified as the relative oil red O stained area. Values represent the mean±standard error of mean (SEM); **p<0.01 vs. WT MSC.
|
IGF-1-induced IGF-1R–Lnk dissociation and Akt/mTOR pathway activation
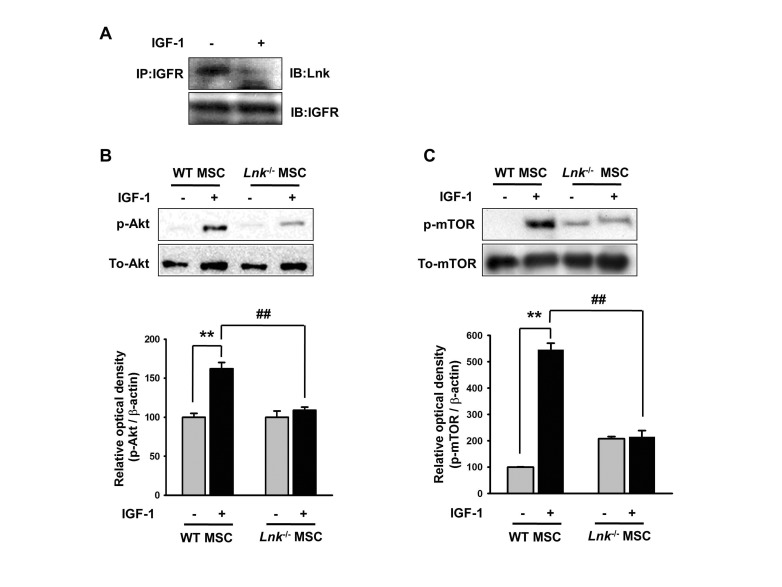 | Fig. 3Insulin-like growth factor-1-mediated dissociation of the insulinlike growth factor-1 receptor–Lnk complex promotes the phosphorylation of Akt/mTOR.(A) Wild-type MSCs were treated with insulin-like growth factor-1 (IGF-1) for 10 min. Coimmunoprecipitation was performed using antibodies against IGF-1 receptor (IGF-1R) and Lnk. (B and C) Wild-type MSCs were treated with IGF-1 for 10 min, and formation of phosphorylated Akt or mTOR was detected by performing western blotting. The lower panels denote the mean±SEM for each condition, as determined from densitometry relative to β-actin; **p<0.01 vs. wild-type MSCs not treated with IGF-1, ##p<0.01 vs. Lnk–/– MSCs treated with IGF-1.
|
Involvement of the Akt/mTOR/PPAR-γ pathway in IGF-1-induced adipogenic differentiation
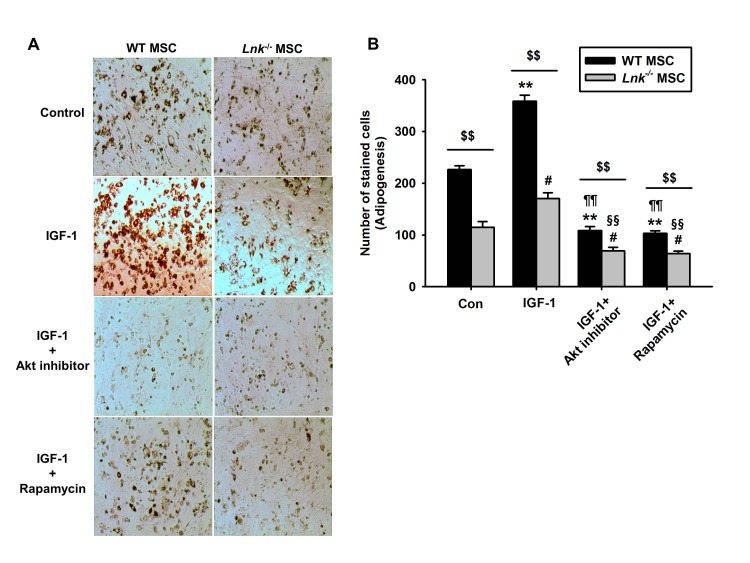 | Fig. 4Involvement of Akt and mTOR signaling in IGF-1-induced differentiation of MSCs.(A) Wild-type (WT) or Lnk–/– MSCs were treated with Akt inhibitor (10 nM) and rapamycin (1 nM). Adipocyte differentiation was induced by incubating the cells in an IGF-1-containing differentiation medium for 14 days and was assessed by staining with oil red O. (B) Adipocyte differentiation was quantified as the number of oil red O stained cells. Values represent the mean±standard error of mean (SEM); **p<0.01 vs. control WT MSC, #p<0.05 and ##p<0.01 vs. control Lnk–/– MSC, ¶¶p<0.01 vs. IGF-treated WT MSC, §§p<0.01 vs. IGF-treated Lnk–/– MSC, $$p<0.01 vs. Lnk–/– MSC treated with PBS (Control), IGF-1 (IGF-1), IGF pretreated with Akt inhibitor (IGF-1+Akt inhibitor), or IGF pretreated Rapamycin (IGF-1+Rapamycin), respectively.
|
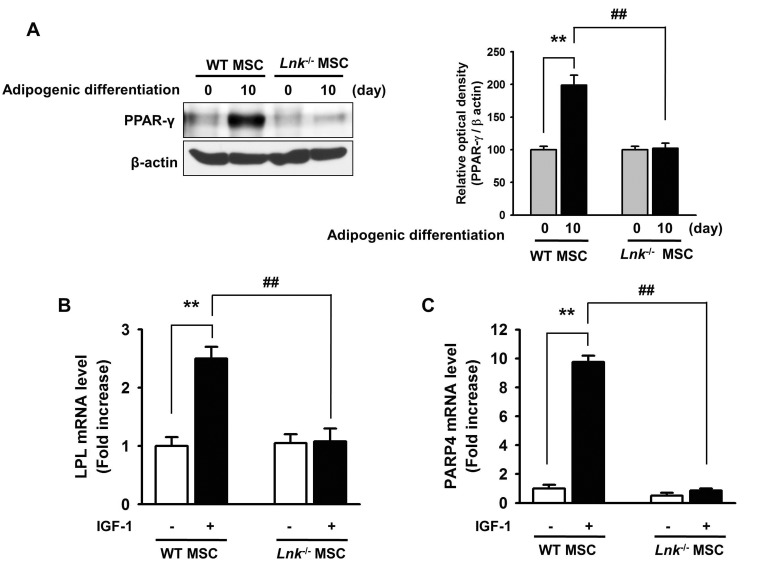 | Fig. 5Expression of PPAR-γ, LPL, and FABP4 during adipogenic differentiation.(A) Adipogenic differentiation of wild-type or Lnk–/– MSCs was induced by incubating the cells in an adipogenic differentiation medium for 10 days. PPAR-γ level was determined by performing western blotting. Bar graph denotes mean±SEM, as determined from densitometry relative to β-actin; **p<0.01 vs. wild-type MSCs (0 day), ##p<0.01 vs. Lnk–/– MSCs (10 day). (B and C) Wild-type or Lnk–/– MSCs were treated with or without 2 ng/ml IGF-1 for 4 days. LPL and FABP4 mRNA expression was quantified by performing quantitative RT-PCR. Values are expressed as mean±SEM; **p<0.01 vs. wild-type MSCs not treated with IGF-1, ##p<0.01 vs. Lnk–/– MSCs treated with IGF-1.
|
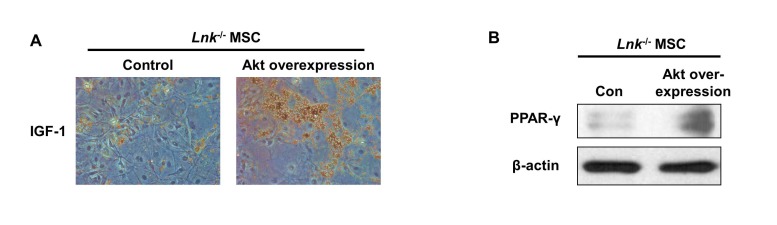 | Fig. 6Adipogenesis and PPAR-γ expression in Lnk–/– MSCs after overexpression of Akt.Akt overexpression in Lnk–/– MSCs were performed by retrovirus-mediated expression of Akt. (A) Adipocyte differentiation was induced by incubating normal or Akt overexpressed Lnk–/– MSCs in an IGF-1-containing differentiation medium for 14 days and was assessed by staining with oil red O. (B) Adipogenic differentiation of normal or Akt overexpressed Lnk–/– MSCs was induced by incubating the cells in an adipogenic differentiation medium for 10 days. PPAR-γ level was determined by performing western blotting.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download