Abstract
N-acetyl-L-cysteine (NAC) and cysteine have been implicated in a number of human neutrophils' functional responses. However, though Ca2+ signaling is one of the key signalings contributing to the functional responses of human neutrophils, effects of NAC and cysteine on intracellular calcium concentration ([Ca2+]i) in human neutrophils have not been investigated yet. Thus, this study was carried out with an objective to investigate the effects of NAC and cysteine on [Ca2+]i in human neutrophils. We observed that NAC (1 µM ~ 1 mM) and cysteine (10 µM ~ 1 mM) increased [Ca2+]i in human neutrophils in a concentration-dependent manner. In NAC pre-supplmented buffer, an additive effect on N-formyl-methionine-leucine-phenylalanine (fMLP)-induced increase in [Ca2+]i in human neutrophils was observed. In Ca2+-free buffer, NAC- and cysteine-induced [Ca2+]i increase in human neutrophils completely disappeared, suggesting that NAC- and cysteine-mediated increase in [Ca2+]i in human neutrophils occur through Ca2+ influx. NAC- and cysteine-induced [Ca2+]i increase was effectively inhibited by calcium channel inhibitors SKF96365 (10 µM) and ruthenium red (20 µM). In Na+-free HEPES, both NAC and cysteine induced a marked increase in [Ca2+]i in human neutrophils, arguing against the possibility that Na+-dependent intracellular uptake of NAC and cysteine is necessary for their [Ca2+]i increasing activity. Our results show that NAC and cysteine induce [Ca2+]i increase through Ca2+ influx in human neutrophils via SKF96365- and ruthenium red-dependent way.
Cysteine, a thiol compound, is a non-essential amino acid. It is synthesized intracellularly from methionine and serine [1]. Synthetic acetylated derivative of cysteine is N-acetyl-L-cysteine (NAC), which was initially employed as a mucolytic agent [2]. Later on, NAC was found to be beneficial beyond mutolytic action. After transporting into cells, NAC is deacetylated to cysteine, which is one of the main building blocks for glutathione (GSH) synthesis [3]. GSH is the major cellular thiol participating in cellular redox reactions and thioether formation [4]. GSH functions as an antioxidant and protects the cells and tissues from the deleterious effects of several free radicals [4]. In these reactions, cysteine moiety of glutathione provides the reactive sulfhydryl group and acts as its functional group. Because of having the sulfhydryl group, NAC can also act directly as a free radical scavenger thus protecting cells from oxidant damage [5]. Biological modulations of NAC have been extensively investigated in diverse sets of experimental systems. In these studies, NAC has been demonstrated to play significant roles in a wide variety of biological processes including inflammation, oxidative stress, cytotoxicity, gene expression, signal transduction, cell proliferation, modulation of apoptosis and carcinogenesis [26].
Neutrophils are an essential component of human innate immune system. Neutrophils respond to invading microbes with chemotaxis to the site of infection, respiratory burst, degranulation and intracellular killing of the microbes [7]. NAC was reported to affect a number of neutrophils functional responses as well. Most of these reports addressed the interference of NAC with the release of reactive oxygen species. NAC at concentrations (25~100 µg/ml or 150~600 µM) inhibited chemiluminescent response of human neutrophils to formylmetionyl-leucyl-phenylalanine (fMLP) [8]. Oral administration of NAC to healthy volunteers resulted in significant reduction of neutrophil chemiluminescence response following opsonized zymosan activation [9]. NAC decreased phorbol-stimulated granulocyte aggregation in a concentration-dependent manner in vitro [10]. NAC at higher concentrations (10–1 M) inhibited human neutrophil chemotaxis [11]. Receptor-mediated phagocytosis of human neutrophils was enhanced by NAC [12], as was antibody-dependent cellular cytotoxicity [13].
Ca2+ signaling is one of the major signalings contributing to these functional responses of human neutrophils. Intracellular free Ca2+ concentration ([Ca2+]i) mediates or essentially regulates important cellular responses in neutrophils including production and release of arachidonic acid products [14], degranulation [1516], respiratory burst [1718], chemotaxis [1920]. Thus, Ca2+ contributes essentially to the function of neutrophils during their defense against bacterial and fungal infections.
However, although NAC functions have been implicated in a number of neutrophil functions, so far no report addressed the effect of NAC on the [Ca2+]i in human neutrophils. Also, there is no report regarding the effect of cysteine on [Ca2+]i in human neutrophils. Thus, in this study NAC and cysteine were characterized with respect to their effect on [Ca2+]i in human neutrophils in in vitro experimental condition.
NAC, cysteine, ruthenium red, fMLP and SKF-96365 were obtained from Sigma-Aldrich Chemical (St. Louis, MO, USA). Fluo-3 AM (acetoxymethyl ester) was from Invitrogen (Grand Island, NY, USA). Solvents and all other buffers and reagents used in this study were of analytical grade.
The study was approved by Ethical Committee of Hallym University. Neutrophils were purified from venous blood of healthy volunteer. In brief, venous blood was collected with peripheral venous puncture and immediately anti-coagulated with 10 U/ml sodium heparin. Then, neutrophils were isolated by density gradient centrifugation in Histopaque-1077, followed by dextran sedimentation. Residual erythrocytes were eliminated with hypotonic lysis. The purity of neutrophils counted by Diff Quik staining was >95% average. Eosinophils were found to be <5%. The viability of neutrophils stained with tryphan blue was >99%.
[Ca2+]i was measured using the fluorescent Ca2+ indicator Fluo-3 AM. Neutrophils were loaded with Fluo-3 AM (4 µM) in HEPES physiologic salt solution (HEPES-PSS) (in mM) (NaCl 140, KCl 5, MgCl2 1, CaCl2 1, Glucose 10, HEPES 10) for 1 h at 37℃. In Na+-free HEPES, NaCl was replaced with equimolar choline chloride. After washing with HEPES-PSS, Fluo-3 AM-loaded neutrophils were re-suspended in HEPES-PSS and plated on 96-well plates at a cell density of 3×106 cells/ml, and then incubated at 37℃ for 10 min for cell stabilization. NAC, cysteine, fMLP or inhibitors were applied at the time points as indicated by the arrowhead in the figures following five minutes pre-read. Traces of [Ca2+]i in Fluo-3 AM-loaded neutrophils were measured with 490 nm/526 nm using Spectramax M2/e fluorescence microplate reader (Molecular Devices). Fluorescent emission readings were recorded every 10 s. Raw fluorescence was subtracted with average fluorescence during 5 min before the addition of stimuli or inhibitor. Changes in [Ca2+]i were expressed as the relative fluorescence intensity of Fluo-3 AM over baseline fluorescence intensity (F/F0). In some analysis, [Ca2+]i following treatment of NAC and cysteine was shown as area under the curve (AUC) analyzed by Graphpad Prism 5.0 (Graphpad software) and was expressed in percentage control (% control).
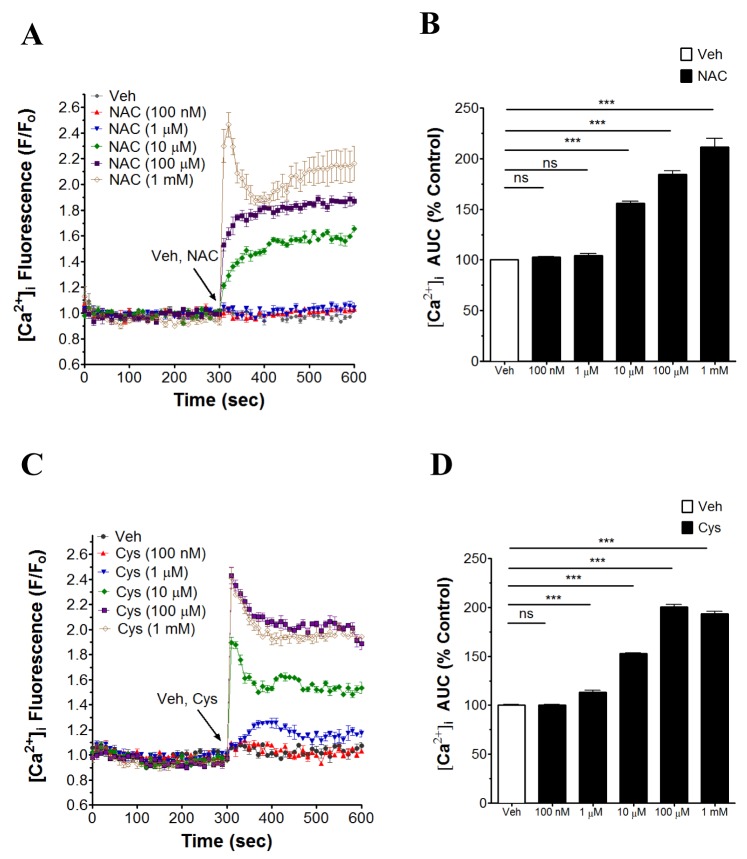
Effects of NAC and cysteine on the [Ca2+]i in human neutrophils have not been reported previously. Thus, the concentration-dependent effect of NAC and cysteine (100 nM~10 mM) on [Ca2+]i in human neutrophils was investigated. As shown in Fig. 1A, B, NAC significantly increased [Ca2+]i in a concentration-dependent manner (from 1 µM~1 mM) in human neutrophils. Concentration-dependent significant increase in [Ca2+]i by cysteine (from 10 µM~1 mM) in human neutrophils also appeared (Fig. 1C, D). However, NAC and cysteine at 10 mM showed an abrupt reversal in the effect with decrease in [Ca2+]i (data not shown).
The primary role of neutrophils is host defense against invading microbes. Neutrophils can be activated by microbial or inflammatory stimuli such as FMLP, C5a, leukotriene B4, platelet activating factor (PAF) and chemokines [21]. Neutrophils express a large number of cell surface receptors [22]. Activation of some of these receptors induces [Ca2+]i increase in neutrophils [22]. Therefore, in the next experiment, we investigated the effects of NAC on fMLP-induced [Ca2+]i increase in human neutrophils. As shown in Fig. 2A~D, NAC (10 µM~1 mM) showed an additive effect on fMLP (1 µM)- induced [Ca2+]i increase in human neutrophils.
The next question was whether NAC- and cysteine-induced [Ca2+]i increase in human neutrophils is from stored calcium mobilization or from calcium influx. Thus, the whole experimentation was operated in Ca2+-free buffer. As shown in Fig. 3A, B, in Ca2+-free buffer supplemented with 1 mM EGTA, NAC- and cysteine-induced [Ca2+]i increase in human neutrophils completely disappeared, suggesting that NAC- and cysteine-mediated increase in [Ca2+]i in human neutrophils occur through Ca2+ influx.
To identify the responsible calcium channel(s) in the NAC-induced [Ca2+]i increase in human neutrophils, several known calcium channel inhibitors were employed. Interestingly, SKF96365, a known inhibitor of Ca2+ release-activated Ca2+ (CRAC) channel, TRPC, TRPV2 and low voltage activated T-type calcium channel [23242526272829] significantly inhibited NAC (100 µM)-induced [Ca2+]i increase in human neutrophils (Fig. 4A, B). Ruthenium red, a common TRPV inhibitor [30] also inhibited NAC (100 µM)-induced [Ca2+]i increase in human neutrophils (Fig. 4C, D). However, 2-APB (10 mM, 100 mM), a range of TRPC [1356] channel inhibitor [27313233343536], mibefradil (1 µM, 10 µM), a T-type calcium channel inhibitor [37], flufenamic acid (100 µM) and clotrimzole (10 µM), known TRPM2 inhibitors [3839], gadolinium chloride (50 µM), a non-specific calcium channel inhibitor [26] and nifedipine (10 µM), an L-type calcium channel inhibitor [40] did not inhibit NAC-induced [Ca2+]i increase in human neutrophils (data not shown). Furthermore, NAC did not increase [Ca2+]i in TRPM2 overexpressed HEK-293 cells (data not shown). Cysteine (10 µM)-induced [Ca2+]i increase in human neutrophils, like NAC, showed the similar sensitivity towards SKF96365 and ruthenium red (Fig. 5A~D).
Na+-dependent transportation of cysteine has been reported in a number of cellular systems including monocytes [41], red blood cells [42], cultured primary astrocytes and neurons [43]. Thus, the next question was asked whether NAC- or cysteine-induced [Ca2+]i increase in human neutrophils is dependent on Na+-dependent transportation of NAC or cysteine inside the cells. To address this query, the whole experiment was performed in Na+-free HEPES. In Na+-free HEPES, NaCl was replaced with the equimolar choline chloride. As seen in Fig. 6A, B, in Na+-free HEPES, NAC (10 µM~1 mM) induced a marked increase in [Ca2+]i in human neutrophils in a concentration dependent manner. Cysteine (1 µM~1 mM) also had similar observation (Fig. 6C, D). This observation suggests that Na+-dependent NAC or cysteine uptake is not necessary to induce [Ca2+]i increase in human neutrophils by NAC and cysteine.
This study shows stimulatory effect of NAC (1 µM~1 mM) and cysteine (10 µM~1 mM) on [Ca2+]i in human neutrophils. Previously, Elferink and de Koster [44] demonstrated that, random migration of rabbit peritoneal neutrophils was enhanced in a chemokinetic way by NAC in the concentration range 10 µM to 400 µM. Their presumption was that Ca2+ influx might have an underlying role for this phenomenon as this migratory effect disappeared in Ca2+-free buffer. In this study, we clearly showed that NAC and cysteine in a concentration-dependent manner increase [Ca2+]i via Ca2+ influx in human neutrophils (Fig. 1A~D). The abrupt reversal in the effect with decrease in [Ca2+]i by NAC and cysteine at 10 mM (data not shown) could be due to the reported toxic effects of NAC at 6~30 mM on human neutrophils [11].
fMLP-stimulated increase in [Ca2+]i plays an important role in the functional responses of human neutrophils such as respiratory burst, chemotaxis, and degranulation [454647]. There is some controversy in the effect of NAC on fMLP-induced respiratory burst in human neutrophils. Sadowska et al showed that preincubation of NAC (1 µM~1 mM) with neutrophils had no effect on fMLP- or PMA-induced respiratory burst, or fMLP-induced migration in human neutrophils [48]. On the other hand, luminol-dependent chemiluminescence in the fMLP-stimulated human neutrophils (first peak) was observed to be suppressed by preincubated NAC at concentrations from 25~100 µg/ml i.e 150~600 µM [8]. In our study, we observed an additive effect of NAC (10 µM~1 mM) on fMLP-stimulated [Ca2+]i in human neutrophils (Fig. 2A~D). In future study, it seems worth clarifying whether NAC-induced additive effect on fMLP-stimulated [Ca2+]i human neutrophils has any modulatory role in its functional responses including respiratory burst.
Notably, both NAC- and cysteine-induced [Ca2+]i increase was inhibited by Ca2+ channel inhibitors SKF96365 and ruthenium red (Figs. 3, 4). The limitation of the study with these Ca2+-channel inhibitors is the broad-specificity of these inhibitors. As for examples, in different studies SKF96365 has been reported to inhibit a wide variety of Ca2+-channels including Ca2+ release-activated Ca2+ (CRAC) channel, TRPC and low voltage activated T-type calcium channel [23242526272829]. Ruthenium red has also been shown to act as a common inhibitor of TRPV channel [26]. However, a calcium channel that is inhibited by both SKF96365 and ruthenium red is TRPV2 [26], which is highly expressed in human neutrophils [49]. Thus, it would be interesting to address in the further study whether NAC- and cysteine-induced [Ca2+]i increase in human neutrophils occurs through TRPV2.
Another interesting observation of this study is that both NAC and cysteine induced a remarkable [Ca2+]i increase in human neutrophils in Na+-free buffer system. Previously, in a number of cellular systems, Na+-dependent transportation of cysteine was reported. Seres et al. showed that only 24% cysteine transport in monocyte is Na+-independent [41]. In red blood cells also cysteine-transport inside the cell was reported as Na+-dependent [42]. The uptake of cysteine in cultured primary astrocytes and neurons was inhibited by around 80% in Na+-free condition [43]. In alveolar type II cells, Na+-dependent transporters XAG and ASC were reported to transport cysteine and cystine [50]. We observed a marked concentration-dependent increase in [Ca2+]i in Na+-free condition by both NAC and cysteine (Fig. 6A-D). This observation excludes the possibility that Na+-dependent uptake of these molecules in human neutrophils is required for these molecules-induced [Ca2+]i increase. Notably, NAC- and cysteine-induced concentration-dependent [Ca2+]i increase in Na+-free condition is very fast and more marked compared to that in the normal Na+ condition with slight differences in effective concentrations (Figs. 1 and 6). These results suggest the extracellular modulatory activity of these molecules to induce [Ca2+]i increase in human neutrophils, which probably occurs through the sulfhydryl moiety present in both NAC and cysteine.
In conclusion, to our knowledge this is the first report showing concentration-dependent increase in [Ca2+]i in human neutrophils by both NAC and cysteine. In this study, we could not demonstrate any definitive mechanism of how NAC and cysteine increase [Ca2+]i in human neutrophils, which is a limitation of the study. In addition, whether this [Ca2+]i increase leads to functional changes of human neutrophils such as chemotaxis, respiratory burst, and degranulation needs to be explored in future study.
ACKNOWLEDGEMENTS
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) (H20150551) and Hallym University Research Fund (HRF-201602-015).
Notes
References
1. Murray RK, Granner DK, Mayes PA, Rodweil VW. Biosynthesis of nutritionally nonessential amino acids. Harper's Biochemistry. 22nd ed. USA: Appleton & Lange;1991. p. 267–269.
2. Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998; 3:114–127. PMID: 9577247.
3. De Flora S, Balansky RM, Bennicelli C, Camoirano A, D'agostini F, Izzotti A, Cesarone CF. Mechanisms of anticarcinogenesis: The example of N-acetylcysteine. In : Ioannides C, Lewis DFV, editors. Drugs Diet and Disease. Mechanistic Approaches to Cancer. Hemel Hempstead, UK: Ellis Horwood;1995. p. 151–203.
4. Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999; 27:916–921. PMID: 10569624.

5. Aitio ML. N-acetylcysteine -- passe-partout or much ado about nothing? Br J Clin Pharmacol. 2006; 61:5–15. PMID: 16390346.

6. De Flora S, Izzotti A, D'agostini F, Balansky RM. Mechanisms of N-acetylcysteine in the prevention of DNA damage and cancer, with special reference to smoking-related end-points. Carcinogenesis. 2001; 22:999–1013. PMID: 11408342.

7. Kobayashi SD, Voyich JM, Burlak C, Deleo FR. Neutrophils in the innate immune response. Arch Immunol Ther Exp (Warsz). 2005; 53:505–517. PMID: 16407783.
8. Paulsen O, Forsgren A. Effects of N-acetylcysteine on human polymorphonuclear leukocytes. APMIS. 1989; 97:115–119. PMID: 2537647.

9. Jensen T, Kharazmi A, Schiøtz PO, Nielsen H, Stenvang Pedersen S, Stafanger G, Koch C, Høiby N. Effect of oral N-acetylcysteine administration on human blood neutrophil and monocyte function. APMIS. 1988; 96:62–67. PMID: 3345250.

10. Bernard GR, Lucht WD, Niedermeyer ME, Snapper JR, Ogletree ML, Brigham KL. Effect of N-acetylcysteine on the pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte function. J Clin Invest. 1984; 73:1772–1784. PMID: 6725559.

11. Kharazmi A, Nielsen H, Schiøtz PO. N-acetylcysteine inhibits human neutrophil and monocyte chemotaxis and oxidative metabolism. Int J Immunopharmacol. 1988; 10:39–46. PMID: 3366508.

12. Ohman L, Dahlgren C, Follin P, Lew D, Stendahl O. N-acetylcysteine enhances receptor-mediated phagocytosis by human neutrophils. Agents Actions. 1992; 36:271–277.
13. Roberts RL, Aroda VR, Ank BJ. N-acetylcysteine enhances antibody-dependent cellular cytotoxicity in neutrophils and mononuclear cells from healthy adults and human immunodeficiency virus-infected patients. J Infect Dis. 1995; 172:1492–1502. PMID: 7594708.

14. Krump E, Pouliot M, Naccache PH, Borgeat P. Leukotriene synthesis in calcium-depleted human neutrophils: arachidonic acid release correlates with calcium influx. Biochem J. 1995; 310:681–688. PMID: 7654211.

15. Nüsse O, Serrander L, Foyouzi-Youssefi R, Monod A, Lew DP, Krause KH. Store-operated Ca2+ influx and stimulation of exocytosis in HL-60 granulocytes. J Biol Chem. 1997; 272:28360–28367. PMID: 9353293.
16. Nüsse O, Serrander L, Lew DP, Krause KH. Ca2+-induced exocytosis in individual human neutrophils: high- and low-affinity granule populations and submaximal responses. EMBO J. 1998; 17:1279–1288. PMID: 9482725.
17. Thelen M, Dewald B, Baggiolini M. Neutrophil signal transduction and activation of the respiratory burst. Physiol Rev. 1993; 73:797–821. PMID: 8415926.

18. Granfeldt D, Samuelsson M, Karlsson A. Capacitative Ca2+ influx and activation of the neutrophil respiratory burst. Different regulation of plasma membrane- and granule-localized NADPH-oxidase. J Leukoc Biol. 2002; 71:611–617. PMID: 11927647.
19. van Kooyk Y, Weder P, Heije K, de Waal Malefijt R, Figdor CG. Role of intracellular Ca2+ levels in the regulation of CD11a/CD18 mediated cell adhesion. Cell Adhes Commun. 1993; 1:21–32. PMID: 7915956.
20. Lawson MA, Maxfield FR. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995; 377:75–79. PMID: 7544874.
21. Tintinger G, Steel HC, Anderson R. Taming the neutrophil: calcium clearance and influx mechanisms as novel targets for pharmacological control. Clin Exp Immunol. 2005; 141:191–200. PMID: 15996182.

22. Futosi K, Fodor S, Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013; 17:638–650. PMID: 23994464.

23. Merritt JE, Armstrong WP, Benham CD, Hallam TJ, Jacob R, Jaxa-Chamiec A, Leigh BK, McCarthy SA, Moores KE, Rink TJ. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990; 271:515–522. PMID: 2173565.
24. Zhu X, Jiang M, Birnbaumer L. Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK)293 cells. Evidence for a non-capacitative Ca2+ entry. J Biol Chem. 1998; 273:133–142. PMID: 9417057.
25. Shlykov SG, Yang M, Alcorn JL, Sanborn BM. Capacitative cation entry in human myometrial cells and augmentation by hTrpC3 overexpression. Biol Reprod. 2003; 69:647–655. PMID: 12700192.
27. Rychkov G, Barritt GJ. TRPC1 Ca2+-permeable channels in animal cells. Handb Exp Pharmacol. 2007; (179):23–52. PMID: 17217049.
28. Bréchard S, Melchior C, Plançon S, Schenten V, Tschirhart EJ. Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium. 2008; 44:492–506. PMID: 18436303.
29. Singh A, Hildebrand ME, Garcia E, Snutch TP. The transient receptor potential channel antagonist SKF96365 is a potent blocker of low-voltage-activated T-type calcium channels. Br J Pharmacol. 2010; 160:1464–1475. PMID: 20590636.

30. Colton CK, Zhu MX. 2-Aminoethoxydiphenyl borate as a common activator of TRPV1, TRPV2, and TRPV3 channels. Handb Exp Pharmacol. 2007; (179):173–187. PMID: 17217057.

31. Gregory RB, Rychkov G, Barritt GJ. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem J. 2001; 354:285–290. PMID: 11171105.
32. Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J Physiol. 2001; 536:3–19. PMID: 11579153.
33. Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002; 16:1145–1150. PMID: 12153982.
34. Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol. 2005; 145:405–414. PMID: 15806115.

35. Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston PA, Heagerty AM, Munsch CM, Bergdahl A, Hultgårdh-Nilsson A, Gomez MF, Porter KE, Hellstrand P, Beech DJ. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006; 98:557–563. PMID: 16439693.

36. Bréchard S, Melchior C, Plançon S, Schenten V, Tschirhart EJ. Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium. 2008; 44:492–506. PMID: 18436303.
37. Sandmann S, Unger T. L- and T-type calcium channel blockade -the efficacy of the calcium channel antagonist mibefradil. J Clin Basic Cardiol. 1999; 2:187–201.
38. Hill K, Benham CD, Mcnulty S, Randall AD. Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology. 2004; 47:450–460. PMID: 15275834.

39. Hill K, Mcnulty S, Randall AD. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn Schmiedebergs Arch Pharmacol. 2004; 370:227–237. PMID: 15549272.

40. Reid K, Guo TZ, Davies MF, Maze M. Nifedipine, an L-type calcium channel blocker, restores the hypnotic response in rats made tolerant to the alpha-2 adrenergic agonist dexmedetomidine. J Pharmacol Exp Ther. 1997; 283:993–999. PMID: 9399968.
41. Seres T, Knickelbein RG, Warshaw JB, Johnston RB Jr. The phagocytosis-associated respiratory burst in human monocytes is associated with increased uptake of glutathione. J Immunol. 2000; 165:3333–3340. PMID: 10975851.

42. Young JD, Wolowyk MW, Jones SE, Ellory JC. Sodium-dependent cysteine transport in human red blood cells. Nature. 1979; 279:800–802. PMID: 450132.

43. Shanker G, Allen JW, Mutkus LA, Aschner M. The uptake of cysteine in cultured primary astrocytes and neurons. Brain Res. 2001; 902:156–163. PMID: 11384608.

44. Elferink JG, de Koster BM. N-acetylcysteine causes a transient stimulation of neutrophil migration. Immunopharmacology. 1998; 38:229–236. PMID: 9506822.

45. Bei L, Hu T, Qian ZM, Shen X. Extracellular Ca2+ regulates the respiratory burst of human neutrophils. Biochim Biophys Acta. 1998; 1404:475–483. PMID: 9739175.
46. Lindemann O, Strodthoff C, Horstmann M, Nielsen N, Jung F, Schimmelpfennig S, Heitzmann M, Schwab A. TRPC1 regulates fMLP-stimulated migration and chemotaxis of neutrophil granulocytes. Biochim Biophys Acta. 2015; 1853:2122–2130. PMID: 25595528.

47. Niessen HW, Kuijpers TW, Roos D, Verhoeven AJ. Release of azurophilic granule contents in fMLP-stimulated neutrophils requires two activation signals, one of which is a rise in cytosolic free Ca2+. Cell Signal. 1991; 3:625–633. PMID: 1786209.
48. Sadowska AM, Manuel-y-keenoy B, Vertongen T, Schippers G, Radomska-Lesniewska D, Heytens E, De Backer WA. Effect of N-acetylcysteine on neutrophil activation markers in healthy volunteers: in vivo and in vitro study. Pharmacol Res. 2006; 53:216–225. PMID: 16384711.

49. Heiner I, Eisfeld J, Lückhoff A. Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium. 2003; 33:533–540. PMID: 12765698.

50. Knickelbein RG, Seres T, Lam G, Johnston RB Jr, Warshaw JB. Characterization of multiple cysteine and cystine transporters in rat alveolar type II cells. Am J Physiol. 1997; 273:L1147–L1155. PMID: 9435569.

Fig. 1
Concentration-dependent effects of NAC and cysteine on [Ca2+]i in human neutrophils.
(A) NAC (100 nM~1 mM) or (C) cysteine (100 nM~1 mM) was treated at 300 s following 5 min pre-read. [Ca2+]i was measured as described in methods and materials. Changes in [Ca2+]i were expressed as the relative fluorescence intensity of Fluo-3 AM over baseline fluorescence intensity (F/F0). (B)&(D) [Ca2+]i following treatment of NAC and cysteine was shown as area under curve (AUC), which was calculated for 900 s (300~1200 s) and was expressed as percentage control (% control). Data were analyzed by Graphpad Prism 5.0 (Graphpad software) using ANOVA. Bonferroni test was used for post-hoc comparison. An average of four (A, B) and three (C, D) independent experiments is shown. ns, no significant difference, **p<0.01, ***p<0.001.
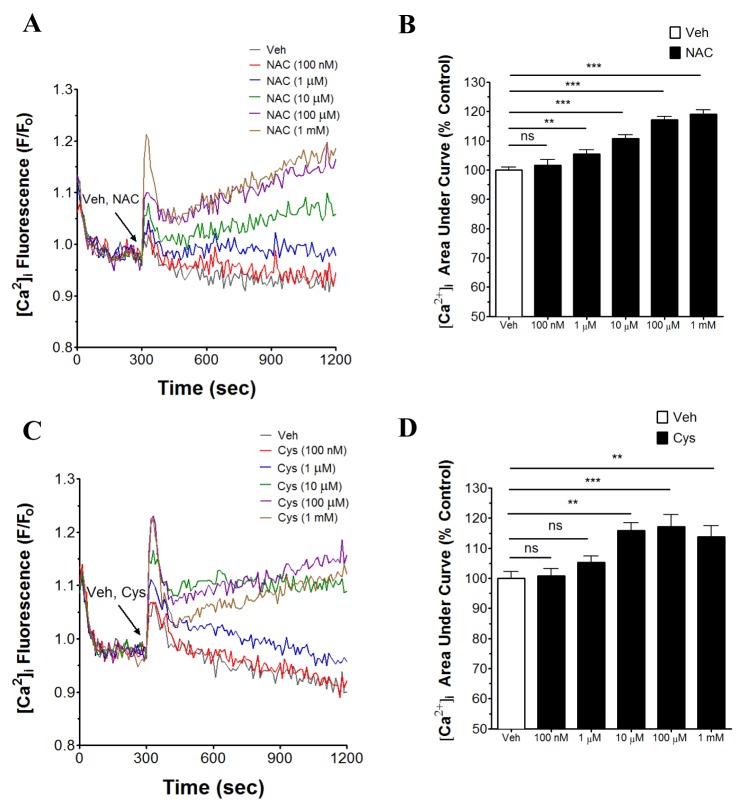
Fig. 2
Effects of NAC and cysteine on fMLP-induced increase in [Ca2+]i in human neutrophils.
Vehicle or NAC at 10 µM, 100 µM and 1 mM (A~C), and fMLP (1 µM) were added at the time points indicated by the arrowheads. Changes in [Ca2+]i were expressed as the relative fluorescence intensity of Fluo-3 AM over baseline fluorescence intensity (F/F0). (D) [Ca2+]i following treatment of vehicle or fMLP (1 µM) was shown as area under curve (AUC), which was calculated for 300 s (900~1200 s) and was expressed as percentage control (% control). Data were analyzed by Graphpad Prism 5.0 (Graphpad software) using ANOVA. Bonferroni test was used for post-hoc comparison. An average of three independent experiments is shown. ***p<0.001.
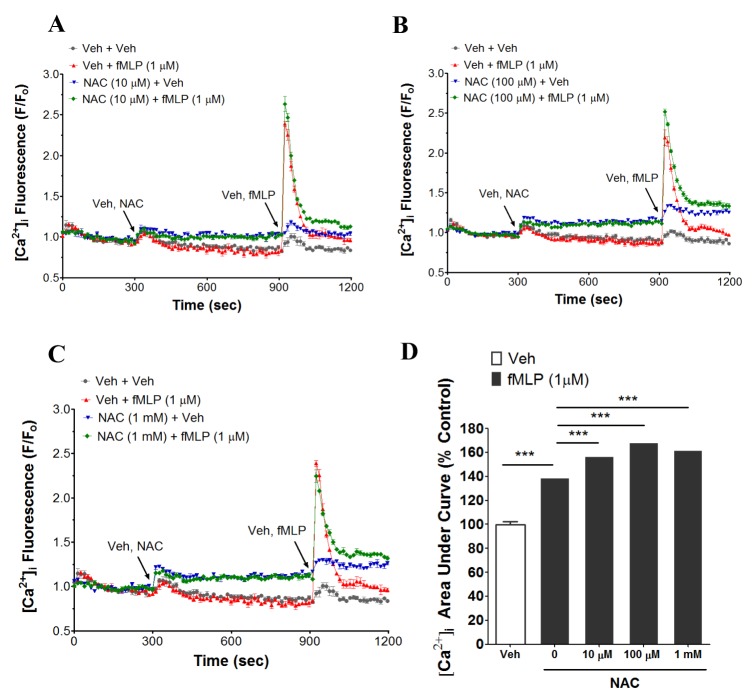
Fig. 3
NAC and cysteine had no effect on [Ca2+]i in human neutrophils in Ca2+-free HEPES buffer.
(A) NAC (100 nM~1 mM) or (B) cysteine (100 nM~1 mM) was treated at 300 s following 5 min pre-read. [Ca2+]i was measured as described in methods and materials. Changes in [Ca2+]i were expressed as the relative fluorescence intensity of Fluo-3 AM over baseline fluorescence intensity (F/F0).
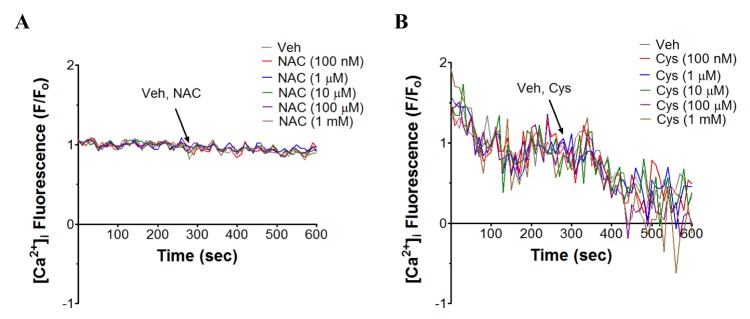
Fig. 4
SKF96365 and ruthenium red inhibit NAC-induced [Ca2+]i increase in human neutrophils.
Ca2+-channel inhibitors (A) SKF96365 (10 µM) and (C) ruthenium red (20 µM), and NAC (100 µM) were added at the time points indicated by the arrowheads. [Ca2+]i was measured as described in methods and materials. Changes in [Ca2+]i were expressed as the relative fluorescence intensity of Fluo-3 AM over baseline fluorescence intensity (F/F0). (B, D) [Ca2+]i following addition of Ca2+-channel inhibitors were shown as area under curve (AUC), which was calculated for 300 s (900~1200 s) and was expressed as percentage control (% control). Data were analyzed by Graphpad Prism 5.0 (Graphpad software) using ANOVA. Bonferroni test was used for post-hoc comparison. An average of three independent experiments is shown. ns, no significant difference, **p<0.01.
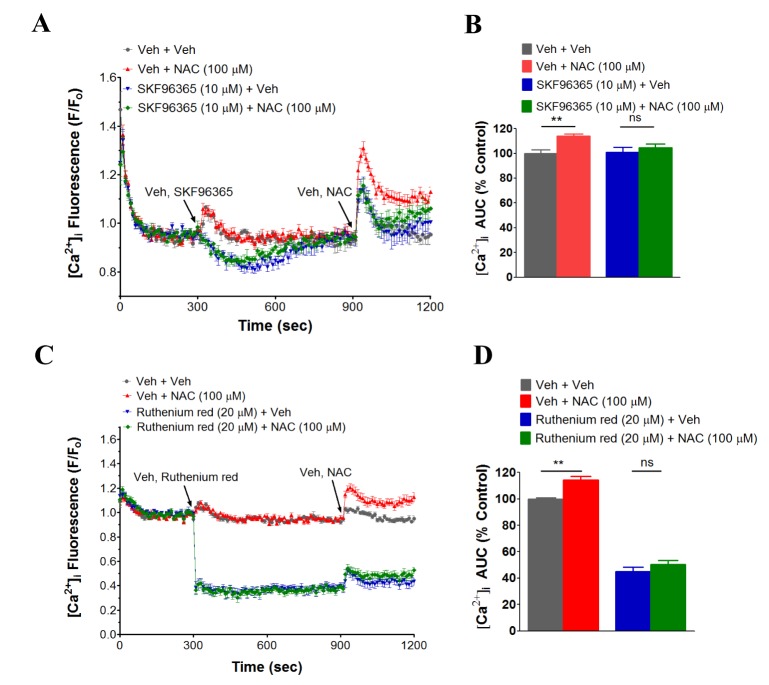
Fig. 5
SKF96365 and ruthenium red inhibit cysteine-induced [Ca2+]i increase in human neutrophils.
Ca2+-channel inhibitors (A) SKF96365 (10 µM) and (C) ruthenium red (20 µM), and cysteine (10 µM) were added at the time points indicated by the arrowheads. [Ca2+]i was measured as described in methods and materials. Changes in [Ca2+]i were expressed as the relative fluorescence intensity of Fluo-3 AM over baseline fluorescence intensity (F/F0). (B, D) [Ca2+]i following addition of Ca2+ channel inhibitors were indicated as area under curve (AUC), which was calculated for 300 s (900~1200 s) and was expressed as percentage control (% control). Data were analyzed by Graphpad Prism 5.0 (Graphpad software) using ANOVA. Bonferroni test was used for post-hoc comparison. An average of three independent experiments is shown. ns, no significant difference, *p<0.05.
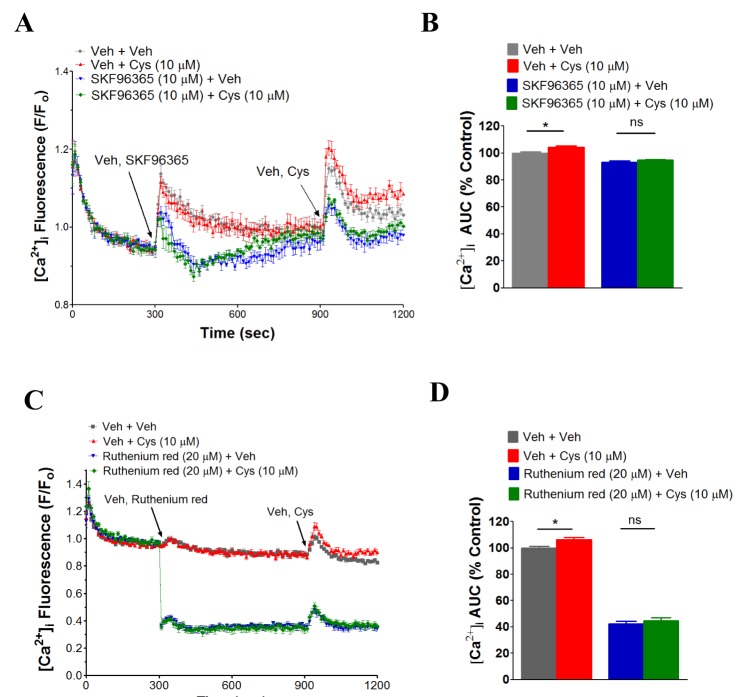
Fig. 6
NAC and cysteine increase [Ca2+]i in human neutrophils in Na+-free HEPES buffer.
(A) NAC (100 nM~1 mM) or (C) cysteine (100 nM~1 mM) was treated at 300 s following 5 min preread. [Ca2+]i was measured as described in methods and materials. Changes in [Ca2+]i were expressed as the relative fluorescence intensity of Fluo-3 AM over baseline fluorescence intensity (F/F0). (B)&(D) [Ca2+]i following treatment of NAC and cysteine was shown as area under curve (AUC), which was calculated for 900 s (300~1200 s) and was expressed as percentage control (% control). Data were analyzed by Graphpad Prism 5.0 (Graphpad software) using ANOVA. Bonferroni test was used for post-hoc comparison. An average of three independent experiments is shown. ns, no significant difference, ***p<0.001.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download