Abstract
Berberine is an isoquinoline alkaloid found in Rhizoma coptidis, and elicits anti-inflammatory effects through diverse mechanisms. Based on previous reports that activating transcription factor-3 (ATF-3) acts as a negative regulator of LPS signaling, the authors investigated the possible involvement of ATF-3 in the anti-inflammatory effects of berberine. It was found berberine concentration-dependently induced the expressions of ATF-3 at the mRNA and protein levels and concomitantly suppressed the LPS-induced productions of proinflammatory cytokines (TNF-α, IL-6, and IL-1β). In addition, ATF-3 knockdown abolished the inhibitory effects of berberine on LPS-induced proinflammatory cytokine production, and prevented the berberine-induced suppression of MAPK phosphorylation, but had little effect on AMPK phosphorylation. On the other hand, the effects of berberine, that is, ATF-3 induction, proinflammatory cytokine inhibition, and MAPK inactivation, were prevented by AMPK knockdown, suggesting ATF-3 induction occurs downstream of AMPK activation. The in vivo administration of berberine to mice with LPS-induced endotoxemia increased ATF-3 expression and AMPK phosphorylation in spleen and lung tissues, and concomitantly reduced the plasma and tissue levels of proinflammatory cytokines. These results suggest berberine has an anti-inflammatory effect on macrophages and that this effect is attributable, at least in part, to pathways involving AMPK activation and ATF-3 induction.
Berberine is an active component of the Chinese medicinal herb Rhizoma coptidis, and has been shown to exhibit a variety of pharmacological effects, which include fever-lowering activity [12]. Notably, berberine also has beneficial effects on metabolic diseases, such as, hyperlipidemia and type 2 diabetes mellitus [34], and has, on a number of occasions, been reported to have anti-inflammatory effects in vitro and in vivo [5678]. For example, berberine reduced COX-2-mediated PGE2 production in oral cancer cell lines, and suppressed neuroinflammation in microglia via AMP-activated protein kinase (AMPK) activation [910].
AMPK acts as a metabolic energy sensor and plays a key role in the regulation of glucose and lipid homeostasis [1112]. It is a heterotrimeric complex composed of a catalytic α subunit and regulatory β- and γ-subunits. When the cellular ATP/AMP ratios are diminished, AMPK is activated by phosphorylation of threonine 172 of its α-subunit [13]. AMPK activation has been reported to protect against metabolic stresses in many studies, and thus, is viewed as a therapeutic target. Furthermore, although the anti-inflammatory effects of AMPK activation are well accepted, the mechanisms underlying AMPK activation appear to be diverse and need clarification.
Of the various stress-induced signaling mediators, activating transcription factor-3 (ATF-3), a member of the ATF/cAMP responsive element binding protein (CREB) family [14], acts as a negative regulator of toll-like receptor 4 (TLR-4), and thus, acts to suppress cellular stresses [1516]. Accordingly, ATF-3 is markedly induced by various stresses, such as, by LPS in endothelial cells, smooth muscle cells, and macrophages [17]. Furthermore, ATF-3 knockout mice were reported to show markedly elevated plasma IL-6 and IL-12b levels after LPS treatment as compared with wild type mice, which confirmed the protective role of ATF-3 on LPS response in vivo [18]. In addition, ATF-3 was shown to attenuate saturated free fatty acid and TLR-4 signaling and macrophage activation in obese adipose tissue in vivo [19].
We hypothesized that berberine might exhibit anti-inflammatory effects in macrophages by inducing ATF-3, and thus, we investigated the possible involvement of ATF-3 in the action of berberine using RAW264.7 murine macrophages and in vivo LPS-induced endotoxemia mouse model.
Male C57BL/6J mice (7 weeks old) were obtained from Orient Bio (Seongnam, Korea), and acclimated for a week before experiments. Animals were housed under specific pathogen-free conditions in an air conditioned room at 23±2℃. Food and water were supplied ad libitum. All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publications, No 8523, revised 2011), and were approved by the Animal Care and Use Committee of Gachon University.
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin, and streptomycin were obtained from GIBCO (Grand Island, NY). LPS (Escherichia Coli 055:B5) and berberine (purity >98%) were from Sigma (St Louis, MO). TNF-α, IL-1β, and IL-6 ELISA kits were from Enzo Life Sciences (New York, NY). Antibodies against phospho-AMPK-α-Thr172, AMPK, phospho-JNK (Thr183/Tyr185), and phospho-p38 MAPK (Thr180/Tyr182) were from Cell Signaling Technology (Danvers, MA). Antibodies against IgG, β-actin, ATF-3, phospho-ERK1/2 (Tyr204), JNK, p38 and ERK1/2 were from Santa Cruz Biotechnology (Santa Cruz, CA).
RAW264.7 murine macrophage cell lines were obtained from the Korean Cell Line Bank and seeded in 12-well plates at a density of 5×105 cells/well in DMEM supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% (v/v) heat-inactivated fetal bovine serum in a humidified 5% CO2/95% air at 37℃. Cells were treated with berberine at the indicated concentrations for 20 hr and then with LPS (10 ng/ml) for 4 hr (ELISA and RT-PCR) and for 30 min (western blot of phosphoprotein). For time dependency of berberine action, cells were treated with 20 µM berberine at the indicated times (0~20 hr), and then with LPS (10 ng/ml) for 4 hr. Final DMSO concentration was 0.5%, which itself had little effect.
Total RNA was isolated from cells using the easy-BLUE Total RNA extraction kit (iNtRON Inc. Korea). Reverse transcription of total RNA (1 µg) was performed using AccuPower RT PreMix (Bioneer Inc. Korea). The sequences of the PCR primers used to amplify TNF-α, IL-6, ATF-3, and GAPDH (the internal standard) were: TNF-α (F)-ATG AGC ACA GAA AGC ATG ATC, TNF-α (R)-TAC AGG CTT GTC ACT CGA ATT; IL-6 (F)-GAG GAT ACC ACT CCC AAC AGA CC, IL-6 (R)-GAG GAT ACC ACT CCC AAC AGA CC; IL-1β (F)-CAG GAT CAG GAC ATG AGC ACC, IL-1β (R)-CTC TGC AGA CTC AAA CTC CAC; ATF-3 (F)-TTG CTA ACC TGA CAC CCT TTG, ATF-3 (R)-CGG TGC AGG TTG AGC ATG TA; GAPDH (F)-TTC ACC ACC ATG GAG AAG GC, GAPDH (R)-GGC ATG GAC TGT GGT CAT GA. Reverse transcription-PCR was performed over 35 amplification cycles (denaturation at 94℃ for 1 min, annealing at 60℃ for 1 min, and extension at 72℃ for 1 min) followed by a 10 min extension at 72℃. PCR reaction mixtures were electrophoresed on 1.5% agarose gel and visualized using Gel red (Elpis Biotech, Seoul) staining under UV light. Relative mRNA abundances were normalized vs. GAPDH.
Cells were lysed in lysis buffer (50 mM HEPES, pH 7.0, 250 mM NaCl, 5 mM EDTA, 0.1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol, 5 mM Na fluoride, 0.5 mM Na orthovanadate, and 5 µg/ml each of leupeptin and aprotinin) for 10 min at 4℃. Following centrifugation at 12,000 rpm and denaturation, 50 µg aliquots of total proteins were loaded into an 8% sodium dodecyl sulfatepolyacrylamide gel, and then transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). Blocked membranes were incubated with anti-ATF-3, anti-AMPK, anti-phospho-AMPKα-Thr172, anti-phospho-ERK-Tyr204, anti-phospho-p38 MAPK-Thr180/Tyr182, anti-p38 MAPK, anti-JNK, anti-phospho-JNK-Thr183/Tyr185, and anti-actin antibodies and then blotted using secondary antibodies conjugated with horseradish peroxidase (HRP). Immunoreactive bands were visualized by enhanced chemiluminescence (Amersham Life Science, Buckinghamshire, UK), and band signals were quantified using UN-SCAN-IT gel 5.1 software (Silk Scientific, Inc., Orem, Utah). Protein concentrations were determined using the Bio-Rad protein assay reagent, according to the manufacturer's instructions.
Concentrations of TNF-α, IL-6 and IL-1β in supernatants of cultured media were measured using ELISA kits. Briefly, supernatants were incubated in coated 96-well plates at room temperature for 2 hr. Plates were washed, and then incubated with detection antibody for 2 hr. After rewashing the plates, conjugate was added for 30 min, followed by the addition of substrate solution. The reaction was stopped with 1N H2SO4 and optical densities were measured at 450 nm using a microplate reader (Perkin Elmer VictorX4, Waltham, MA).
Raw 264.7 cells were maintained in DMEM medium containing 10% fetal bovine serum, and 5×105 cells/well in 12-well plates were transfected with α1-AMPK siRNA, ATF-3 siRNA, or negative control siRNA (Santa Cruz, CA) (100 nM/well) using 6 µl of Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) in OPTI MEM medium for 6 hr. Medium was then replaced with DMEM containing 10% FBS and 18 hr later, cells were treated with berberine (10 and 20 µM) for 20 hr and then with 10 ng/ml LPS for 30 min (western blot of phosphoprotein) and for 4 hr (ELISA). Efficiencies of ATF-3 and AMPK knockdowns were determined by Western blotting and by RT-PCR.
A subcellular proteome extraction kit (Calbiochem, Inc. San Diego, CA) was used to extract cytosolic and nuclear fractions of RAW264.7 cells. Briefly, cells were incubated with 20 µM berberine for 20 hrs, exposed to LPS (10 ng/ml) for 4 hr, washed twice with PBS, resuspended in 150 µl of ice-cold Extraction I containing 0.75 µl of protease inhibitor mixture, and then incubated for 10 min at 4℃ with gentle agitation. The suspensions obtained were centrifuged at 1000×g for 10 min at 4℃. Supernatants were referred to as cytosolic fraction. Pellets were resuspended in 150 µl of ice-cold Extraction II (for membrane fractions) containing 0.75 µl of protease inhibitor mixture, and incubated for 30 min at 4℃. Mixtures were then centrifuged at 6000×g at 4℃, and the supernatants obtained were referred to as membrane fraction. Remaining pellets were re-suspended in ice-cold Extraction buffer IIII with 0.75 µl of protease inhibitor mixture and incubate for 10 min at 4℃ under gentle agitation. Mixtures were then centrifuged at 8800×g at 4℃ and supernatants were transferred to the tube as a nuclear fraction.
C57BL/6 mice were orally administered vehicle (saline, 200 µl/20 g, n=7) or berberine (100 or 200 mg/kg/day, n=8 each dose) for 3 days twice daily. On day 4, LPS (20 mg/kg, i.p.) was injected 3 hours after last berberine administration. One hour later, mice were anesthetized with diethyl ether and whole blood was collected by cardiac puncture. Plasma was obtained by whole blood centrifugation and stored at -20℃ until assayed. Lungs and spleens were isolated and immediately frozen, and stored in liquid nitrogen until assayed. All tissues were homogenized and then lysed using a Prep-sol (iNtRON, Korea) prior to analysis.
RAW264.7 macrophages were pretreated with various concentrations of berberine for 20 hr and then stimulated with 10 ng/ml LPS for 4 hr. Although LPS markedly increased proinflammatory cytokine production as compared with control cells, berberine (10~100 µM) concentration-dependently suppressed this production (Fig. 1A). Likewise, the mRNA expressions of proinflammatory cytokines were concentration-dependently reduced by berberine pretreatment (Fig. 1B). Suppression of LPS-stimulated TNF-α and IL-6 production by berberine (20 µM) was also time dependent, maximum effects being achieved at 20 hr incubation (Fig. 1C).
We next examined the involvement of ATF-3 in the berberine-mediated suppression of LPS-induced proinf lammatory cytokines. Along with the suppression of proinflammatory cytokine production, berberine concentration-dependently increased ATF-3 mRNA expression (2.1 to 4.9 fold vs. controls, and 1.4 to 2.3 fold vs. LPS alone). Similarly, as has been previously reported, ATF-3 protein levels were increased by LPS [1518], and were further enhanced by berberine pretreatment (3.4 to 4.1 fold vs. controls, and 2.4 to 2.9 fold vs. LPS alone) (Fig. 1D).
Since ATF-3 is known to translocate into nucleus upon stimulation, we further examined the effects of berberine on nuclear translocation of ATF-3 after subcellular fractionation. As shown in Fig. 1E, β-tubulin, a cytosolic marker was detected in the cytosolic fraction, whereas the expression of lamin B1 was restricted in the nucleus fraction, indicating that subcellular fractionation was successful. On the other hand, ATF-3 was primarily detected in nucleus fraction, and berberine (20 µM) consistently super-induced protein levels of ATF-3.
To elucidate the role of ATF-3 induction by berberine, we examined the effects of ATF-3 knockdown on the anti-inflammatory effects of berberine. Transfection of ATF-3 siRNA decreased ATF-3 mRNA and protein expressions by ~60% as compared with control siRNA treatment (Fig. 2A). ATF-3 siRNA transfection completely blocked the inhibitory effects of berberine on proinflammatory cytokine production (Fig. 2B), suggesting that ATF-3 is critical for the suppressive actions of berberine on LPS-induced proinflammatory cytokine production in macrophages.
MAPK signaling is known to participate in the inflammatory effect of LPS [20]. Thus, we examined the effects of berberine on MAPK phosphorylation and the effects of ATF-3 siRNA transfection on the effects of berberine on MAPKs. As shown in Fig. 3A, berberine suppressed the phosphorylations of JNK, ERK, and p38 MAPK, and these suppressions were completely blocked by ATF-3 knockdown. On the other hand, Thr172 phosphorylation of AMPKα was little affected by ATF-3 siRNA treatment (Fig. 3B). These results suggest berberine induces ATF-3 induction and suppresses MAPK activation, and that AMPK activation by berberine maybe an upstream mediator of ATF-3 induction.
To examine the signaling pathway of ATF-3 induction by berberine further, we checked the effects of AMPK knockdown on berberine-induced ATF-3 induction. As shown in Fig. 4A, AMPK siRNA reduced the mRNA and protein expressions of AMPK to 36% and 46% of control levels, respectively. As was expected, AMPK siRNA blunted the effects of berberine on proinflammatory cytokine production (Fig. 4B). Likewise, ATF-3 induction and MAPK deactivation by berberine were also blocked by AMPK knockdown (Fig. 4C). In view of the observation that ATF-3 knockdown had little effect on berberine-induced AMPK phosphorylation, these results suggest sequential signaling pathways in the actions of berberine, i.e., AMPK phosphorylation → ATF-3 induction → inactivation of MAPK.
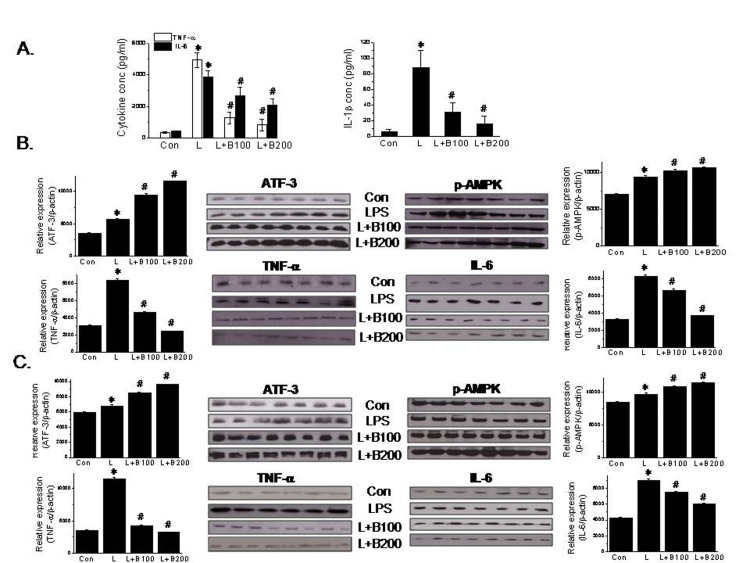
The involvement of ATF-3 induction in the anti-inflammatory action of berberine was further investigated in vivo using a LPS-induced septic shock mouse model. LPS injection significantly increased the plasma levels of proinflammatory cytokines (Fig. 5A); its effects peaked at 1 hr post-injection and then declined rapidly (results not shown). However, oral administration of berberine (100 or 200 mg/kg, bid) for 3 days significantly reduced the plasma levels of proinflammatory cytokines (Fig. 5A). Consistent with our in vitro results, ATF-3 levels were elevated in lungs and spleens (both major contributors to macrophage production) of berberine-treated animals, and phosphorylated AMPK levels were elevated and the protein levels of inflammatory markers were diminished (Figs. 5B and C). Taken together, it appears that AMPK-mediated ATF-3 induction is an important component of the anti-inflammatory effects of berberine in murine macrophages.
The present study was undertaken to investigate the possible involvement of ATF-3 in the anti-inflammatory action of berberine. Our findings suggest that the inhibition of LPS-induced proinflammatory cytokine (TNF-α, IL-1β and IL-6) production by berberine is due, at least in part, to ATF-3 induction in an AMPK-dependent manner. This was confirmed by our in vivo study using LPS-induced endotoxemia model as we found proinflammatory cytokine levels were reduced in plasma and tissues upon berberine administration. These results provide novel evidence that ATF-3 is involved in the anti-inflammatory effects of berberine, and extends the possible application of ATF-3 modulators to the treatment of inflammation-related diseases.
Recently published evidence suggests type 2 diabetes patients display features of inflammation, and that chronic low-grade inflammation is associated with pathogenesis of metabolic diseases [2122]. Accordingly, it seems a series of proinflammatory cytokines produced in local circumstances might be pivotal players in the development of insulin resistance and the subsequent development of type 2 diabetes. In keeping with this notion, various approaches targeting the intervention of inflammatory process are being developed and are widely expected to progress the treatment of metabolic diseases, particularly during early disease stages [23]. However, clinical trial results on agents targeting inflammation have not less than successful to date. It appears that the origin of the inflammatory process underlying type 2 diabetes derives largely from macrophage infiltration into obese adipose tissues and their contributions to inflammatory conditions, possibly via a phenotypic change to M1 pro-inflammatory polarization [2425]. Hence, understanding of the complexities of the actions of macrophages and those of pharmacological modulators on macrophage functions would probably provide clues regarding the treatments of metabolic disorders.
AMPK has been identified as a molecular target of metformin, a widely used anti-diabetic agent [2627], although other mechanisms have also been reported to be responsible for anti-diabetic action of metformin [2829]. On the other hand, AMPK activation and various subsequent events have been reported to contribute substantially to its anti-inflammatory effects. For example, eNOS activation in endothelial cells [30] and iNOS inhibition in myocytes, adipocytes, and mouse bone marrow-derived macrophages [31] have all been associated with AMPK activation all anti-inflammatory effects.
ATF-3 is a stress-activated transcription factor, but its role depends on the cellular context, that is, it can be protective or detrimental. It has been previously reported that ATF-3 negatively regulates TLR signaling [18], and our results support the notion that ATF-3 protects cells from stress. Furthermore, pharmacological induction of ATF-3 may provide beneficial effects against inflammation and possibly insulin resistance. The action of metformin provides additional example of ATF-3 induction, as the super-induction of ATF-3 has been shown to be implicated in the anti-inflammatory effects of metformin in murine primary macrophages [32]. In accordance with a previous report that showed ATF-3 inhibits inflammation in different tissues [33], our study suggests that ATF-3 induction by berberine suppresses proinflammatory cytokine up-regulation by LPS. Furthermore, it describes a novel anti-inflammatory mechanism of berberine, that is, one involving the induction ATF-3 expression. This mechanism is supported by the observations of others, for example, the suppression of the LPS-induced expression of MMP in human monocytes by IFN-γ was found to be mediated by the superinduction of ATF-3 and suppression of AP-1 in a STAT1-dependent manner [34], and similarly, Stearns et al. found the IL-10-induced inhibition of MMP2 expression in human prostate CPTX-1532 cells was ATF-3 dependent [35].
With regard to the mechanistic link between ATF-3 induction and inhibition of the productions of TNF-α and IL-6, Hashimoto et al. showed ATF-3 binds directly to the promoter region of IL-6, and thus, suppresses its transcription [16], and prior reports showed that ATF-3 acts as a negative regulator of IL-6 [18]. Interestingly, heat shock-induced suppression of LPS-induced IL-6 expression was found to be mediated via heat shock transcription factor 1 (HSF1)-mediated ATF-3 overexpression [36], and in other studies, ATF-3 was also shown to repress TNF-α transcription by binding to the activator protein-1 site of TNF-α promoter [3237], which led to complex formation with histone deacetylase, chromatin remodeling, and the transcriptional inhibition of proinflammatory cytokines. All these reports are consistent with our observation that ATF-3 superinduction decreases the expressions of proinflammatory cytokine markers.
Because AMPK was found to participate in the anti-inflammatory effects of berberine in murine macrophages, we investigated the relationship between berberine-induced AMPK activation and ATF-3 induction. Whereas, AMPK siRNA prevented berberine-mediated ATF-3 induction and the suppression of proinflammatory cytokine production, AICAR (a direct AMPK activator) induced ATF-3 expression. On the other hand, ATF-3 siRNA did not affect berberine-induced AMPK phosphorylation but blocked the suppression of proinflammatory cytokine production by berberine. Thus, AMPK activation by berberine appears to occur prior to ATF-3 induction and to play an essential step in the up-regulation of ATF-3.
Of the multiple signaling pathways involved in the action of LPS, MAPK activation, including the activations of p38, ERK (extracellular signal-regulated kinase), and JNK (c-Jun NH2 terminal kinase), is a prerequisite of inflammatory cytokine production [20]. In the present study, as was expected, the LPS-induced phosphorylations of all three of these MAPKs were inhibited by berberine, and these inhibitions were prevented by ATF-3 siRNA and by AMPK siRNA. Together, these results suggest ATF-3 acts as a downstream mediator of AMPK activation and an upstream mediators of the MAPK pathway. However, the detailed mechanisms as to how AMPK activation by berberine leads to ATF-3 induction remain to be disclosed.
The involvement of ATF-3 induction in the action of berberine was confirmed in vivo. In line with our in vitro observations, the anti-inflammatory effects of berberine in vivo were found to be associated with ATF-3 up-regulation in macrophage-rich tissues. An intraperitoneal injection of LPS boosted plasma levels of TNF-α and IL-6 within 1 hr, and the oral administration of berberine for 3 consecutive days to LPS-induced endotoxemic mice reduced plasma levels of proinflammatory cytokines and concomitantly increased AMPK phosphorylation and ATF-3 expression in lung and spleen tissues. Taken together, these in vivo results concur with our in vitro finding that the anti-inflammatory action of berberine is mediated via ATF-3 induction in macrophages. Interestingly, Suganami et al. reported transgenic mice overexpressing human ATF-3 in macrophages had lower levels of TNF-α and IL-6 mRNA in adipose tissues and peritoneal macrophages, which suggests ATF-3 overexpression attenuates macrophage activation in vivo [19]. Nonetheless, it is possible that other mechanistic pathways are involved in berberine action.
Summarizing, berberine was found to upregulate ATF-3 expression in murine macrophages, and consequently to reduce TLR signaling for proinflammatory cytokine production. These studies provide novel mechanisms of berberine's anti-inflammatory action, i.e. ATF-3 induction. Furthermore, our findings suggest that the pharmacological modulation of ATF-3 expression or activation provides a potential strategy for treating inflammation-related diseases.
ACKNOWLEDGEMENTS
This research was supported by the intramural research fund of Gachon University (project no. GCU-2015-5106) and by a grant from the Korean Health Technology R&D Project through the Korean Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1135).
References
1. Schmeller T, Latz-Brüning B, Wink M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry. 1997; 44:257–266. PMID: 9004542.

2. Kuo CL, Chou CC, Yung BY. Berberine complexes with DNA in the berberine-induced apoptosis in human leukemic HL-60 cells. Cancer Lett. 1995; 93:193–200. PMID: 7621428.

3. Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S, Wu J, Wang Y, Li Z, Liu J, Jiang JD. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004; 10:1344–1351. PMID: 15531889.

4. Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006; 55:2256–2264. PMID: 16873688.

5. Choi BH, Ahn IS, Kim YH, Park JW, Lee SY, Hyun CK, Do MS. Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp Mol Med. 2006; 38:599–605. PMID: 17202835.

6. Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009; 296:E955–E964. PMID: 19208854.

7. Kim KW, Ha KT, Park CS, Jin UH, Chang HW, Lee IS, Kim CH. Polygonum cuspidatum, compared with baicalin and berberine, inhibits inducible nitric oxide synthase and cyclooxygenase-2 gene expressions in RAW 264.7 macrophages. Vascul Pharmacol. 2007; 47:99–107. PMID: 17553752.

8. Lee CH, Chen JC, Hsiang CY, Wu SL, Wu HC, Ho TY. Berberine suppresses inf lammatory agents-induced interleukin-1beta and tumor necrosis factor-alpha productions via the inhibition of IkappaB degradation in human lung cells. Pharmacol Res. 2007; 56:193–201. PMID: 17681786.
9. Lu DY, Tang CH, Chen YH, Wei IH. Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J Cell Biochem. 2010; 110:697–705. PMID: 20512929.

10. Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004; 203:127–137. PMID: 14732220.

11. Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998; 67:821–855. PMID: 9759505.

12. Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004; 279:47898–47905. PMID: 15371448.

13. Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003; 144:5179–5183. PMID: 12960015.

14. Cai Y, Zhang C, Nawa T, Aso T, Tanaka M, Oshiro S, Ichijo H, Kitajima S. Homocysteine-responsive ATF3 gene expression in human vascular endothelial cells: activation of c-Jun NH(2)-terminal kinase and promoter response element. Blood. 2000; 96:2140–2148. PMID: 10979959.

15. Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999; 7:321–335. PMID: 10440233.
16. Hashimoto Y, Zhang C, Kawauchi J, Imoto I, Adachi MT, Inazawa J, Amagasa T, Hai T, Kitajima S. An alternatively spliced isoform of transcriptional repressor ATF3 and its induction by stress stimuli. Nucleic Acids Res. 2002; 30:2398–2406. PMID: 12034827.

17. Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001; 2:599–609. PMID: 11483993.

18. Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Roach JC, Kennedy K, Hai T, Bolouri H, Aderem A. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006; 441:173–178. PMID: 16688168.

19. Suganami T, Yuan X, Shimoda Y, Uchio-Yamada K, Nakagawa N, Shirakawa I, Usami T, Tsukahara T, Nakayama K, Miyamoto Y, Yasuda K, Matsuda J, Kamei Y, Kitajima S, Ogawa Y. Activating transcription factor 3 constitutes a negative feedback mechanism that attenuates saturated Fatty acid/toll-like receptor 4 signaling and macrophage activation in obese adipose tissue. Circ Res. 2009; 105:25–32. PMID: 19478204.

20. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002; 298:1911–1912. PMID: 12471242.

21. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821–1830. PMID: 14679177.

22. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–1808. PMID: 14679176.

23. Burcelin R. Regulation of metabolism: a cross talk between gut microbiota and its human host. Physiology (Bethesda). 2012; 27:300–307. PMID: 23026753.

24. Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci. 2006; 1084:89–117. PMID: 17151295.

25. Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009; 58:2574–2582. PMID: 19690061.

26. Kaneto H, Kawamori D, Nakatani Y, Gorogawa S, Matsuoka TA. Oxidative stress and the JNK pathway as a potential therapeutic target for diabetes. Drug News Perspect. 2004; 17:447–453. PMID: 15514704.

27. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001; 108:1167–1174. PMID: 11602624.

28. El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000; 275:223–228. PMID: 10617608.

29. Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000; 348:607–614. PMID: 10839993.

30. Huang NL, Chiang SH, Hsueh CH, Liang YJ, Chen YJ, Lai LP. Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IkappaB-alpha degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int J Cardiol. 2009; 134:169–175. PMID: 18597869.
31. Pilon G, Dallaire P, Marette A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J Biol Chem. 2004; 279:20767–20774. PMID: 14985344.
32. Kim J, Kwak HJ, Cha JY, Jeong YS, Rhee SD, Kim KR, Cheon HG. Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction. J Biol Chem. 2014; 289:23246–23255. PMID: 24973221.

33. Nilsson R, Bajic VB, Suzuki H, di Bernardo D, Björkegren J, Katayama S, Reid JF, Sweet MJ, Gariboldi M, Carninci P, Hayashizaki Y, Hume DA, Tegner J, Ravasi T. Transcriptional network dynamics in macrophage activation. Genomics. 2006; 88:133–142. PMID: 16698233.

34. Ho HH, Antoniv TT, Ji JD, Ivashkiv LB. Lipopolysaccharide-induced expression of matrix metalloproteinases in human monocytes is suppressed by IFN-gamma via superinduction of ATF-3 and suppression of AP-1. J Immunol. 2008; 181:5089–5097. PMID: 18802113.
35. Stearns ME, Kim G, Garcia F, Wang M. Interleukin-10 induced activating transcription factor 3 transcriptional suppression of matrix metalloproteinase-2 gene expression in human prostate CPTX-1532 Cells. Mol Cancer Res. 2004; 2:403–416. PMID: 15280448.
36. Takii R, Inouye S, Fujimoto M, Nakamura T, Shinkawa T, Prakasam R, Tan K, Hayashida N, Ichikawa H, Hai T, Nakai A. Heat shock transcription factor 1 inhibits expression of IL-6 through activating transcription factor 3. J Immunol. 2010; 184:1041–1048. PMID: 20018623.

37. Whitmore MM, Iparraguirre A, Kubelka L, Weninger W, Hai T, Williams BR. Negative regulation of TLR-signaling pathways by activating transcription factor-3. J Immunol. 2007; 179:3622–3630. PMID: 17785797.

Fig. 1
Effects of berberine on LPS-induced proinflammatory cytokine production and ATF-3 expression.
Raw264.7 cells were treated with different concentrations of berberine (10~100 µM) for 20 hr and then with 10 ng/ml of LPS for 4 hr. Cell culture media were collected, and TNF-α, IL-1β, and IL-6 levels were determined by ELISA (A); Proinflammatory cytokine mRNA expressions were determined by RT-PCR (B); and the effect of berberine on ATF-3 expression was determined by RT-PCR and western blot (D). The time dependency of berberine action was examined after incubation of RAW264.7 cells with 20 µM berberine for different incubation times (0~20 hr), followed by 4 hr exposure of LPS (10 ng/ml) (C). Nuclear translocation of ATF-3 after berberine treatment was examined by subcellular fractionation followed by western blot (E). The experiment was repeated twice in triplicate, and results are expressed as means±SEMs. *p<0.05 vs. control. #p<0.05 vs. LPS alone.
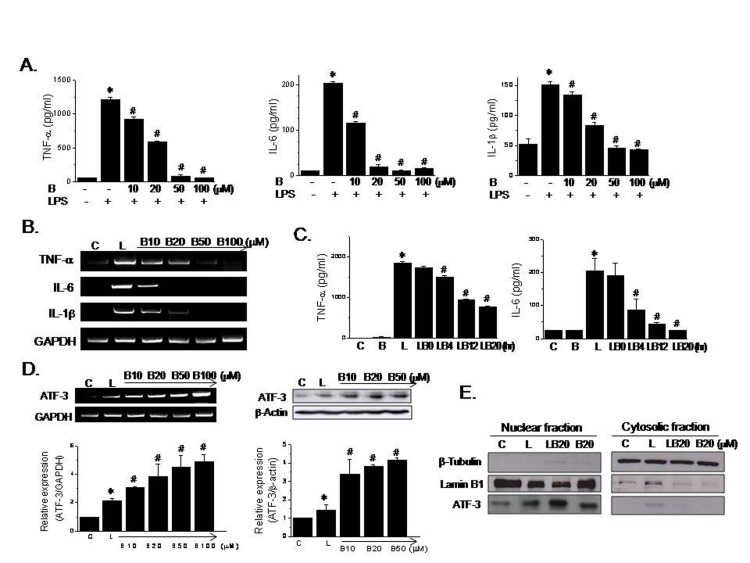
Fig. 2
Effects of ATF-3 knockdown on the berberine-induced suppression of proinflammatory cytokine production by LPS.
ATF-3 siRNA (100 nM/well) or control siRNA was transfected into Raw264.7 cells, and extents of ATF-3 knockdown were assessed by RT-PCR and western blot (A). Cells transfected with control siRNA or ATF-3 siRNA were treated with berberine (10 or 20 µM) for 20 hr and then with LPS (10 ng/ml) for 4 hr. Levels of TNF-α, IL-1β, and IL-6 in culture medium were determined by ELISA (B). The experiment was repeated twice in triplicate, and results are expressed as means±SEMs. *p<0.05 vs. control. #p<0.05 vs. LPS alone. †p<0.05 vs. control siRNA.
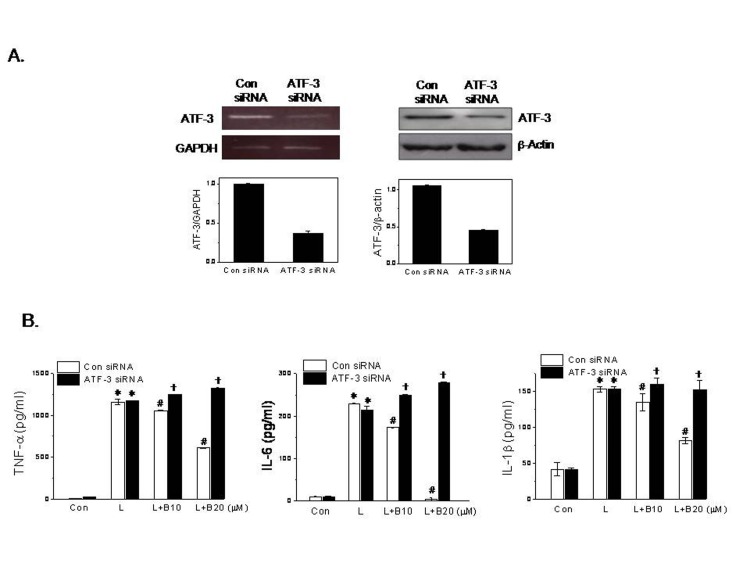
Fig. 3
Effects of ATF-3 knockdown on the berberine-induced suppression of MAPK phosphorylation by LPS.
Control siRNA or ATF-3 siRNA (100 nM/well) was transfected into Raw264.7 cells, which were then treated with berberine for 20 hr and then with LPS (10 ng/ml) for 30 min. Phosphorylated levels of p38 (Thr180/Tyr182), ERK (Tyr204), JNK (Thr183/Tyr185) (A), and AMPK-α (Thr172) (B) were determined by western blot. Densitometric analysis was carried out using UN-SCAN-IT gel ver. 5.1 software. The experiment was repeated three times and representative results are shown. *p<0.05 vs. control. #p<0.05 vs. LPS alone. †p<0.05 vs. control siRNA.
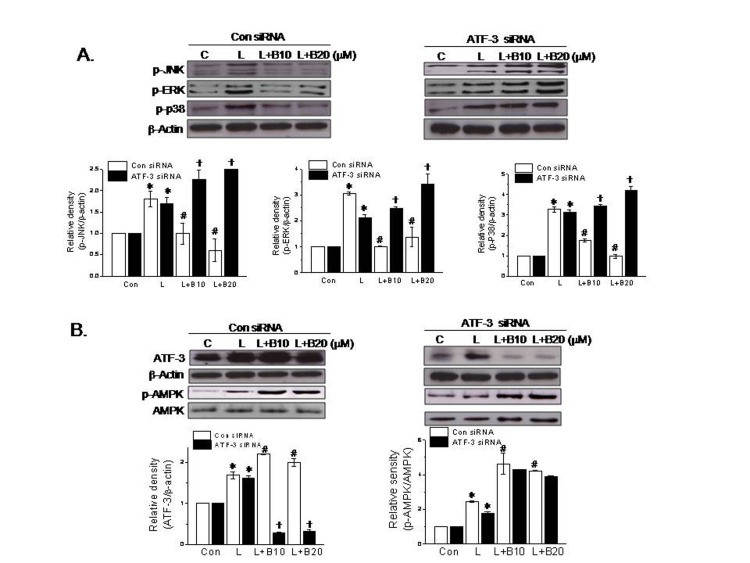
Fig. 4
Effects of AMPK knockdown on LPS-induced proinflammatory cytokine production.
AMPK siRNA (100 nM/well) or control siRNA was transfected into Raw26.4 7 cells, and extents of AMPK knockdown were determined by RT-PCR and western blot (A). Cells transfected with control or AMPK siRNA were treated with berberine for 20 hr and then with LPS for 4 hr (B) and for 30 min (C). Levels of TNF-α, IL-1β, and IL-6 in culture media were determined by ELISA (B). Phosphorylated levels of p38 (Thr180/Tyr182), ERK (Tyr204), JNK (Thr183/Tyr185), and AMPK-α (Thr172) were determined by western blot, and densitometric analysis was carried out by using UN-SCAN-IT gel ver. 5.1 software (C). The experiment was repeated three times, and representative results are shown. *p<0.05 vs. control. #p<0.05 vs. LPS alone. †p<0.05 vs. control siRNA.
Fig. 5
In vivo effects of berberine in the LPS-induced septic shock mouse model.
C57BL/6J mice (7 weeks old, male) were administered berberine (100 or 200 mg/kg, n=8 per dose) or vehicle (0.5% CMC, n=7) orally for 3 consecutive days, and on the 4th day administered LPS (20 mg/kg, i.p.). One hour after LPS administration, plasma was obtained by cardiac puncture, and plasma levels of TNF-α, IL-1β, and IL-6 were determined by ELISA (A). Lung and spleens were obtained immediately after cardiac puncture and homogenized. The expression patterns of ATF-3, p-AMPK (Thr172), TNF-α, and IL-6 in spleen (B) and lung tissues (C) were determined by western blot; densitometric analysis was carried out using UN-SCAN-IT gel ver. 5.1 software. The experiment was repeated twice and results are expressed as means±SEMs. *p<0.05 vs. control. #p<0.05 vs. LPS alone.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download