Abstract
Early life neuronal exposure to environmental toxicants has been suggested to be an important etiology of neurodegenerative disease development. Perfluorohexanesulfonate (PFHxS), one of the major perfluoroalkyl compounds, is widely distributed environmental contaminants. We have reported that PFHxS induces neuronal apoptosis via ERK-mediated pathway. Imperatorin is a furanocoumarin found in various edible plants and has a wide range of pharmacological effects including neuroprotection. In this study, the effects of imperatorin on PFHxS-induced neuronal apoptosis and the underlying mechanisms are examined using cerebellar granule cells (CGC). CGC were isolated from seven-day old rats and were grown in culture for seven days. Caspase-3 activity and TUNEL staining were used to determine neuronal apoptosis. PFHxS-induced apoptosis of CGC was significantly reduced by imperatorin and PD98059, an ERK pathway inhibitor. PFHxS induced a persistent increase in intracellular calcium, which was significantly blocked by imperatorin, NMDA receptor antagonist, MK801 and the L-type voltage-dependent calcium channel blockers, diltiazem and nifedipine. The activation of caspase-3 by PFHxS was also inhibited by MK801, diltiazem and nifedipine. PFHxS-increased ERK activation was inhibited by imperatorin, MK801, diltiazem and nifedipine. Taken together, imperatorin protects CGC against PFHxS-induced apoptosis via inhibition of NMDA receptor/intracellular calcium-mediated ERK pathway.
Neurodegenerative diseases have attracted more attention as the senior population worldwide increases. Among the etiologies, environmental pollutants have been suggested as potential causal factors [12]. Several epidemiological and animal studies suggest a positive correlation between early exposure to environmental toxicants and increased risk of development of neurodegenerative diseases including Alzheimer's disease and Parkinson's disease in later life [3].
Perfluorohexanesulfonate (PFHxS), one of the major perfluoroalkyl compounds (PFCs), has been widely used in a numerous consumer and industrial applications, such as coatings of carpets, fabrics and food container, paint formulation, and fire-fighting foams [4]. Due to its extreme stability, PFHxS accumulates in environment and has been detected in the serum of the general population, as well as in occupational workers. More importantly, PFHxS is found in higher levels in children than in adults [5]. This raises a serious concern regarding PFHxS' effect on health, particularly its possible neurotoxic effects. Recent reports demonstrate that neonatal exposure to PFHxS causes behavioral and cognitive disturbances in adult mice [6].
Neuronal apoptosis is a common final step of numerous neuropathological conditions regardless of cause. In particular, neuronal apoptosis during developmental periods is responsible for neurobehavioral disturbances [7]. We have previously reported that PFHxS induced apoptosis of rat cerebellar granule cells (CGC) on postnatal day 14 (PND 14) condition [8]. The proliferation and differentiation of cerebellum in rodents occurs very actively during the first three weeks after birth, which corresponds to the last trimester in human, a brain growth-spurt period [9]. Therefore, CGC of PND 14 has been widely used for developmental neurotoxicity studies. Multiple signaling pathways, including N-methyl-D-aspartic acid (NMDA) receptor and mitogen-activated protein kinases (MAPKs) pathways, regulate neuronal apoptosis [10111213].
The NMDA receptor is an ionotropic glutamate receptor and known to be involved in diverse neuronal functions by mediating excitatory neuronal transmissions [14]. The activation of the NMDA receptor induces Ca2+ influx, which play a critical role in physiological neuronal functions including synaptic plasticity, memory formation and learning. However, over-activation of the NMDA receptor induces an excessive Ca2+ influx [1516], resulting in excitotoxic neuronal damage observed in many neuropathological conditions [1718192021].
MAPKs including ERK, p38 MAPK and JNK are the most widespread signaling molecules involved in diverse cellular responses, including cell survival and death [222324]. In the previous studies, we have reported that PFHxS-induced apoptosis of rat pheochromocytoma cells (PC12 cells) is regulated by NMDA receptor-mediated ERK 1/2 pathway [825].
Imperatorin, a bioactive furanocoumarin, is found in various edible plants including Angelica dahurica and Angelica archangelica [2627] used in traditional medicine for treatment of various diseases, including neurological diseases [28]. Wang et al. report that imperatorin protects neuronal cells from apoptosis induced by hypoxia re-oxygenation [29]. A recent animal study also showed that imperatorin improved cognitive impairment induced by scopolamine [30]. Moreover, pharmacokinetic studies have shown that imperatorin is easily distributed in the brain after oral administration [3132], suggesting its potential for neurological disease treatment. In the present study, we examined the protective effects of imperatorin on PFHxS-induced neuronal apoptosis and underlying mechanisms using rat CGC on PND 14.
Imperatorin was purchased from ChromaDex (Irvine, CA, USA). Dulbecco's modified eagle's medium (DMEM) and fetal bovine serum (FBS) were obtained from Invitrogen (Carlsbad, CA, USA). Insulin, transferrin, γ-amino butyric acid, poly-L-lysine, cytosine arabinoside, MK801, diltiazem, and nifedipine were from Sigma-Aldrich (St. Louis, MO, USA). The ERK and phospho-ERK antibodies were from Cell Signaling Technology (Beverly, MA) and GAPDH antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
CGC were prepared as described previously [33]. Briefly, cerebella were isolated from 7-day old Sprague-Dawley rat. CGC were obtained by digestion with trypsin-DNase and then plated on poly-L-lysine coated culture plates in DMEM supplemented with 10% FBS, 25 mM KCL, 5 µg/ml of insulin, transferrin, and γ-amino butyric acid. After 40 h of culture, cells were treated with 5 µM of cytosine arabinoside to prevent growth of non-neuronal cells. Cells were maintained for 7 days in culture and used for experiments. Protocols involving animals were approved by Catholic University of Daegu Animal Ethics Committee (approval No. 2013-1218-CU-AEC-22-Y).
Cell viability was measured using MTS assay kit (Promega, Medison, WI, USA) containing 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and phenazine ethosulfate. Cells were seeded in 96-well plate (1.2×104 cells/well) and treated with 20 µl of MTS solution. After 2 h of incubation at 37℃, the absorbance was detected at 490 nm by a microplate reader (Bio-Rad, Hercules, CA, USA).
Caspase-3 activity was measured by using Caspase-Glo 3/7 assay kit (Promega). Briefly, cells grown on 96-well plates were treated with a luminogenic substrate containing the DEVD sequence and the relative light units were measured using a Plus LB 96 V luminometer (Berthold Detection System, Oak Ridge, TN, USA). The data were represented as fold increase over the control.
DNA fragmentation was detected with terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-nick end-labeling (TUNEL) assay kit (DeadEnd TM fluorometric TUNEL system, Promega) according to the manufacturer's protocol. In brief, cells grown on poly-L-lysine coated chamber slides were fixed with a freshly prepared 4% paraformaldehyde for 25 min at 4℃, washed 2 times with ice-cold PBS and permeabilized with 0.2% Triton X-100 in PBS on ice for 5 min. The TdT and fluorescein-12-dUTP reactions were performed for 1 h at 37℃ in a humidified box, and then mounted with propidium iodide (PI) to stain all cells for counterstaining. TUNEL- and PI-positive cells were imaged using a fluorescence microscope (U-LH 100-3, Olympus, Tokyo, Japan). Both TUNEL – and PI-positive cells were counted and the numbers of apoptotic cells (TUNEL-positive cells) were expressed as a percentage of the total number of cells (PI-positive cells).
The intracellular [Ca2+] was measured using calcium detection kit according to the manufacturer's instructions (Abcam, Cambridge, MA, USA). Briefly, cells grown on 60 mm dishes were homogenized and centrifuged at 21,000 g for 15 min at 4℃. The supernatant was collected and reacted with chromogenic reagent. The absorbance of formed chromophore was measured at 575 nm.
Cell lysates were separated by SDS-PAGE gel and transferred to nitrocellulose membrane. After blocking with 5% non-fat dry milk, the blots were incubated with primary antibodies for phospho-ERK1/2 and ERK1/2 (Cell signaling, Beverly, MA) and then, reacted with a peroxidase-conjugated secondary antibody. The protein bands were detected by the Super Signal (Pierce, Rockford, IL). The density of respective bands was analyzed by the Chemi-Doc XRS imaging system (Bio-Rad, Hercules, CA). The membranes were reprobed with anti-GAPDH antibody, which was used as loading control.
Cells were treated with different concentrations (0~1000 nM) of imperatorin for 24 h and cell viability was measured by MTS assay to examine its cytotoxic effects. Imperatorin did not affect cell viability up to 1000 nM (Fig. 1A). In the previous study, we showed that PFHxS-induced CGC apoptosis was completely blocked by caspase-3 inhibition and ERK pathway inhibition [8]. The effects of imperatorin on neuronal apoptosis induced by PFHxS were evaluated by measuring caspase-3 activity. Imperatorin reduced PFHxS-increased caspase-3 activity in dose-dependent manner with maximum inhibition at 500 nM. In support of our previous study, PFHxS-increased caspase-3 activity was blocked by PD98059, an ERK pathway inhibitor (Fig. 1B). The effect of imperatorin on neuronal apoptosis was further evaluated by TUNEL staining and visualized under a microscope. PFHxS (300 µM) treatment for 24 h increased the number of TUNEL positive cells about 10 folds compared to the DMSO in control. This increase was significantly reduced by 44% and 89% by 500 nM of imperatorin and the casepase-3 inhibitor, AC-DEVD-CHO, respectively (Fig. 2A and 2B).
We have previously reported that the activation of the NMDA receptor and subsequent increase in Ca2+ influx are involved in PFHxS-induced apoptosis of neuronal differentiated PC12 cells [8]. Similarly, caspase-3 activation by PFHxS in CGC was reduced significantly by a NMDA receptor antagonist, MK801, and L-type voltage-gated calcium channel (L-VGCC) blockers, diltiazem and nifedipine (Fig. 3A). The effect of MK801 on PFHxS-induced apoptosis was confirmed by TUNEL assay (Fig. 2A and 2B). PFHxS-increased number of TUNEL-positive cells was decreased by 68% by MK801. Therefore, the involvement of NMDA receptor in the protective effects of imperatorin was examined. NMDA treatment for 15 min increased caspase-3 activity about 2-fold, which was significantly reduced by MK801, diltiazem and nifedipine. Imperatorin also significantly decreased NMDA-induced caspase3 activity (Fig. 3B).
The involvement of NMDA receptor in the action of imperatorin was further examined by measuring intracellular Ca2+ concentration ([Ca2+]i). PFHxS significantly increased the level of [Ca2+]i with maximum increase at 1 h. The increase was maintained for up to 2 h and then gradually returned to basal at 24 h (Fig. 3C). The PFHxS-induced elevation of [Ca2+]i was almost completely blocked by MK801, diltiazem and nifedipine. Imperatorin (100 and 500 nM) also significantly reduced the level of [Ca2+]i increased by PFHxS. (Fig. 3D). These results indicate that PFHxS-induced persistent Ca2+ influx occurs primarily via the NMDA receptor and L-VGCCs and the anti-apoptotic effects of imperatorin involve the inhibition of NMDA receptor activation and intracellular Ca2+ increase.
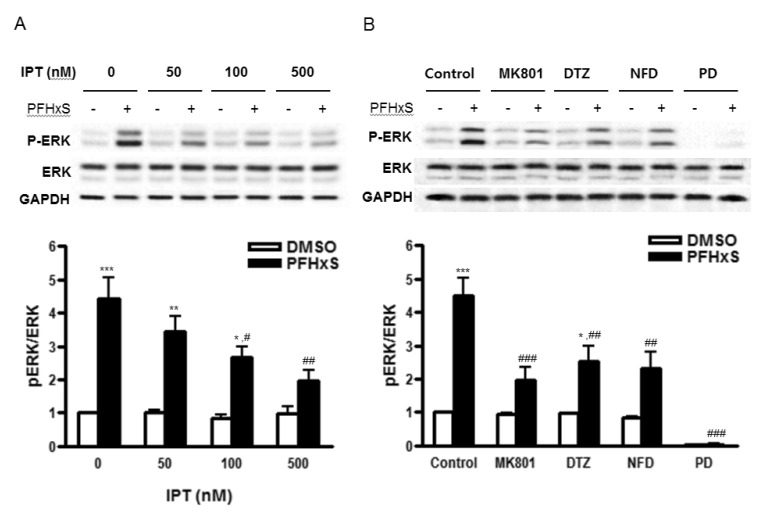
We have previously shown that PFHxS-induced CGC apoptosis was mediated by ERK pathway [8]. The effect of imperatorin on PFHxS-induced ERK activation was examined by Western blot. The PFHxS-induced ERK phosphorylation was inhibited significantly by imperatorin in a dose-dependent manner (Fig. 4A). The ERK phosphorylation was also blocked significantly by MK801, diltiazem and nifedipine (Fig. 4B), indicating that ERK activation is regulated by PFHxS-increased intracellular Ca2+ and the protective effects of imperatorin involve ERK inhibition.
PFHxS is one of the major PFCs. Although perfluorooctane sulfonate (PFOS) among PFCs has been the most widely used and studied, PFOS has been replaced with PFHxS in various industrial applications due to the potential toxic effects of PFOS, including neurotoxicity. However, we and others have shown that PFHxS also induce neurotoxic effects similar to PFOS [6825]. Cumulative studies have shown that PFHxS along with other PFCs are found in cord blood and breast milk, as well as maternal serum, suggesting perinatal exposure to PFHxS [343536]. The perinatal brain is highly susceptible to environmental insults and neurologic damage during this development period could be detrimental and irreversible [3738]. Over the past decade, numerous plants and their constituents have been investigated for their therapeutic potential for neurological disorder. In the present study, we examined the beneficial effects of imperatorin on PFHxS-induced neuronal apoptosis using rat CGC on PND14. Imperatorin significantly reduced PFHxS-induced CGC apoptosis at 50~1000 nM without having cytotoxic effects at these concentrations.
Intracellular calcium is a key signaling molecule involved in many neuronal functions and the levels of intracellular calcium are finely regulated under physiological conditions. However, an overload of intracellular calcium causes ion homeostasis imbalance, resulting in neuronal damage. Among the routes of Ca2+ entry, the NMDA receptor plays the most important role in neuronal excitotoxicity [39]. Overstimulation of the NMDA receptor leads to excess Ca2+ and membrane depolarization that causes L-VGCC activation and in turn, further increase [Ca2+]i by Ca2+-induced Ca2+ release from intracellular calcium storage [4041].
In the present study, PFHxS-induced CGC apoptosis and persistent elevation of [Ca2+]i, were almost completely blocked by the NMDA receptor antagonist and L-VGCC blockers. Imperatorin significantly reduced PFHxS-increased [Ca2+]i and NMDA-induced apoptosis of CGC in a concentration-dependent manner. These results suggest that PFHxS-induced apoptosis of CGC was mediated by increased [Ca2+]i via the NMDA receptor and L-VGCC. Further, the neuronal protective effects of imperatorin were attributed to its inhibition of NMDA receptor-mediated intracellular calcium overload. The inhibitory effect of imperatorin on [Ca2+]i has been reported by others. The antihypertensive effect of imperatorin was mediated by reduction of Ca2+ influx via L-VGCC [4243]. Wang et al. have published opposing reports that imperatorin at 3 µM increases Ca2+ influx, possibly by N-and P/Q-type Ca2+ channel activation, in hippocampal nerve terminals [44]. This suggests that imperatorin has differential effects on calcium channel subtypes depending on cell type. Future studies to elaborate Ca2+ influx regulation by imperatorin in PFHxS-induced apoptosis of CGC are necessary.
The roles of MAPKs including ERK, JNK and p38 MAPK in neuronal apoptosis have been extensively studied. In the previous studies, we have shown that PFHxS-induced apoptosis was selectively inhibited by an ERK pathway inhibitor [825]. The activation of ERK is regulated by multiple upstream signaling molecules including the NMDA receptor and calcium [45464748]. We have also reported that PFHxS-increased ERK activation in PC12 cells was attenuated by MK801 [25]. In accordance with these observations, the NMDA receptor antagonist and L-VGCC blockers significantly reduced PFHxS-increased ERK phosphorylation in CGC. Imperatorin also inhibited PFHxS-induced ERK phosphorylation in a concentration-dependent manner. These results indicate that PFHxS-induced ERK activation is mediated by intracellular calcium and the protective effects of imperatorin involve ERK inhibition. Similar to our observations, the preventive effect of imperatorin on acute lung injury model involved ERK inhibition [49]. However, the opposite effect of imperatorin on ERK pathway has been reported [50]. In that study, imperatorin increased ERK activation resulting in enhanced bone formation in osteoblast, suggesting that the effect of imperatorin on ERK pathway is diverse in different experimental models.
In summary, present study provides the mechanisms responsible for the protective effects of imperatorin against PFHxS-induced apoptosis of CGC. PFHxS induces CGC apoptosis by intracellular Ca2+-mediated ERK activation and imperatroin exerts its protective effects through suppression of intracellular calcium and ERK activation. With evidence of its high bloodbrain barrier permeability, imperatorin may be a useful therapeutic candidate for treatment of neurological disorders involving excitotoxic neuronal damage.
ACKNOWLEDGEMENT
This work was supported by the grant of Research Institute of Medical Science, Catholic University of Daegu (2014).
References
1. Caudle WM, Guillot TS, Lazo CR, Miller GW. Industrial toxicants and Parkinson's disease. Neurotoxicology. 2012; 33:178–188. PMID: 22309908.

2. Trojsi F, Monsurro MR, Tedeschi G. Exposure to environmental toxicants and pathogenesis of amyotrophic lateral sclerosis: state of the art and research perspectives. Int J Mol Sci. 2013; 14:15286–15311. PMID: 23887652.
3. Fox DA, Grandjean P, de Groot D, Paule MG. Developmental origins of adult diseases and neurotoxicity: epidemiological and experimental studies. Neurotoxicology. 2012; 33:810–816. PMID: 22245043.

4. Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011; 7:513–541. PMID: 21793199.

5. Gump BB, Wu Q, Dumas AK, Kannan K. Perfluorochemical (PFC) exposure in children: associations with impaired response inhibition. Environ Sci Technol. 2011; 45:8151–8159. PMID: 21682250.

6. Viberg H, Lee I, Eriksson P. Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology. 2013; 304:185–191. PMID: 23287389.

7. Olney JW. New insights and new issues in developmental neurotoxicology. Neurotoxicology. 2002; 23:659–668. PMID: 12520755.

8. Lee YJ, Choi SY, Yang JH. PFHxS induces apoptosis of neuronal cells via ERK1/2-mediated pathway. Chemosphere. 2014; 94:121–127. PMID: 24125707.

9. Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973; 48:757–767. PMID: 4796010.

10. Bonfoco E, Leist M, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Cytoskeletal breakdown and apoptosis elicited by NO donors in cerebellar granule cells require NMDA receptor activation. J Neurochem. 1996; 67:2484–2493. PMID: 8931482.

11. Tenneti L, D'Emilia DM, Troy CM, Lipton SA. Role of caspases in N-methyl-D-aspartate-induced apoptosis in cerebrocortical neurons. J Neurochem. 1998; 71:946–959. PMID: 9721720.

12. Yamagishi S, Matsumoto T, Numakawa T, Yokomaku D, Adachi N, Hatanaka H, Yamada M, Shimoke K, Ikeuchi T. ERK1/2 are involved in low potassium-induced apoptotic signaling downstream of ASK1-p38 MAPK pathway in cultured cerebellar granule neurons. Brain Res. 2005; 1038:223–230. PMID: 15757638.

13. Vacotto M, Coso O, Fiszer de Plazas S. Programmed cell death and differential JNK, p38 and ERK response in a prenatal acute hypoxic hypoxia model. Neurochem Int. 2008; 52:857–863. PMID: 18077057.

14. Crowder JM, Croucher MJ, Bradford HF, Collins JF. Excitatory amino acid receptors and depolarization-induced Ca2+ influx into hippocampal slices. J Neurochem. 1987; 48:1917–1924. PMID: 2437250.
15. Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989; 36:106–112. PMID: 2568579.
16. Urushitani M, Nakamizo T, Inoue R, Sawada H, Kihara T, Honda K, Akaike A, Shimohama S. N-methyl-D-aspartate receptor-mediated mitochondrial Ca2+ overload in acute excitotoxic motor neuron death: a mechanism distinct from chronic neurotoxicity after Ca2+ influx. J Neurosci Res. 2001; 63:377–387. PMID: 11223912.
17. Auzmendi J, Gonzalez N, Girardi E. The NMDAR subunit NR2B expression is modified in hippocampus after repetitive seizures. Neurochem Res. 2009; 34:819–826. PMID: 18751892.

18. Choi DW. Glutamate receptors and the induction of excitotoxic neuronal death. Prog Brain Res. 1994; 100:47–51. PMID: 7938533.
19. Hsieh MH, Gu SL, Ho SC, Pawlak CR, Lin CL, Ho YJ, Lai TJ, Wu FY. Effects of MK-801 on recognition and neurodegeneration in an MPTP-induced Parkinson's rat model. Behav Brain Res. 2012; 229:41–47. PMID: 22227506.

20. Mota SI, Ferreira IL, Rego AC. Dysfunctional synapse in Alzheimer's disease - a focus on NMDA receptors. Neuropharmacology. 2014; 76:16–26. PMID: 23973316.

21. Olivares D, Deshpande VK, Shi Y, Lahiri DK, Greig NH, Rogers JT, Huang X. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer's disease, vascular dementia and Parkinson's disease. Curr Alzheimer Res. 2012; 9:746–758. PMID: 21875407.

22. Fan J, Gladding CM, Wang L, Zhang LY, Kaufman AM, Milnerwood AJ, Raymond LA. P38 MAPK is involved in enhanced NMDA receptor-dependent excitotoxicity in YAC transgenic mouse model of Huntington disease. Neurobiol Dis. 2012; 45:999–1009. PMID: 22198502.

23. Poddar R, Paul S. Novel crosstalk between ERK MAPK and p38 MAPK leads to homocysteine-NMDA receptor-mediated neuronal cell death. J Neurochem. 2013; 124:558–570. PMID: 23176034.

24. Xiao L, Feng C, Chen Y. Glucocorticoid rapidly enhances NMDAevoked neurotoxicity by attenuating the NR2A-containing NMDA receptor-mediated ERK1/2 activation. Mol Endocrinol. 2010; 24:497–510. PMID: 20160127.

25. Lee YJ, Choi SY, Yang JH. NMDA receptor-mediated ERK 1/2 pathway is involved in PFHxS-induced apoptosis of PC12 cells. Sci Total Environ. 2014; 491-492:227–234. PMID: 24534200.

26. Baek NI, Ahn EM, Kim HY, Park YD. Furanocoumarins from the root of angelica dahurica. Arch Pharm Res. 2000; 23:467–470. PMID: 11059825.
27. Lia HB, Chen F. Preparative isolation and purification of bergapten and imperatorin from the medicinal plant cnidium monnieri using high-speed counter-current chromatography by stepwise increasing the flow-rate of the mobile phase. J Chromatogr A. 2004; 1061:51–54. PMID: 15633744.
28. Howes MJ, Perry NS, Houghton PJ. Plants with traditional uses and activities, relevant to the management of Alzheimer's disease and other cognitive disorders. Phytother Res. 2003; 17:1–18. PMID: 12557240.

29. Wang N, Wu L, Cao Y, Wang Y, Zhang Y. The protective activity of imperatorin in cultured neural cells exposed to hypoxia reoxygenation injury via anti-apoptosis. Fitoterapia. 2013; 90:38–43. PMID: 23856091.

30. Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I, Biala G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology (Berl). 2015; 232:931–942. PMID: 25189792.

31. Lili W, Yehong S, Qi Y, Yan H, Jinhui Z, Yan L, Cheng G. In vitro permeability analysis, pharmacokinetic and brain distribution study in mice of imperatorin, isoimperatorin and cnidilin in Radix Angelicae Dahuricae. Fitoterapia. 2013; 85:144–153. PMID: 23353658.

32. Wang Y, Wang N, Wu L, Lu W, Zhang Y. Simultaneous determination of four components in baige capsule by HPLC: application to pharmacokinetics and tissue distribution of normal and middle cerebral artery occlusion rats. Biomed Chromatogr. 2014; 28:541–547. PMID: 24122939.

33. Yang JH, Kodavanti PR. Possible molecular targets of halogenated aromatic hydrocarbons in neuronal cells. Biochem Biophys Res Commun. 2001; 280:1372–1377. PMID: 11162682.

34. Cariou R, Veyrand B, Yamada A, Berrebi A, Zalko D, Durand S, Pollono C, Marchand P, Leblanc JC, Antignac JP, Le Bizec B. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ Int. 2015; 84:71–81. PMID: 26232143.

35. Maisonet M, Calafat AM, Marcus M, Jaakkola JJ, Lashen H. Prenatal exposure to perfluoroalkyl acids and serum testosterone concentrations at 15 years of age in female ALSPAC study participants. Environ Health Perspect. 2015; 123:1325–1330. PMID: 26034840.

36. Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Basterrechea M, Grimalt JO, Jimenez AM, Kraus T, Schettgen T, Sunyer J, Vrijheid M. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ Res. 2015; 142:471–478. PMID: 26257032.

37. Porterfield SP. Vulnerability of the developing brain to thyroid abnormalities: environmental insults to the thyroid system. Environ Health Perspect. 1994; 102(Suppl 2):125–130. PMID: 7925183.

38. Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. EPICure Study Group. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005; 90:F134–F140. PMID: 15724037.

39. Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002; 298:846–850. PMID: 12399596.
40. Fagni L, Chavis P, Ango F, Bockaert J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000; 23:80–88. PMID: 10652549.
41. Sukhareva M, Smith SV, Maric D, Barker JL. Functional properties of ryanodine receptors in hippocampal neurons change during early differentiation in culture. J Neurophysiol. 2002; 88:1077–1087. PMID: 12205130.

42. He JY, Zhang W, He LC, Cao YX. Imperatorin induces vasodilatation possibly via inhibiting voltage dependent calcium channel and receptor-mediated Ca2+ influx and release. Eur J Pharmacol. 2007; 573:170–175. PMID: 17662269.
43. Zhang Y, Cao Y, Wang Q, Zheng L, Zhang J, He L. A potential calcium antagonist and its antihypertensive effects. Fitoterapia. 2011; 82:988–996. PMID: 21679750.

44. Wang SJ, Lin TY, Lu CW, Huang WJ. Osthole and imperatorin, the active constituents of cnidium monnieri (L.) cusson, facilitate glutamate release from rat hippocampal nerve terminals. Neurochem Int. 2008; 53:416–423. PMID: 18951936.

45. Shiflett MW, Balleine BW. Contributions of ERK signaling in the striatum to instrumental learning and performance. Behav Brain Res. 2011; 218:240–247. PMID: 21147168.

46. Webb IC, Coolen LM, Lehman MN. NMDA and PACAP receptor signaling interact to mediate retinal-induced scn cellular rhythmicity in the absence of light. PLoS One. 2013; 8:e76365. PMID: 24098484.

47. Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+, and calmodulin. Cell Signal. 2002; 14:649–654. PMID: 12020764.
48. Qian W, Zhu J, Mao C, Liu J, Wang Y, Wang Q, Liu Y, Gao R, Xiao H, Wang J. Involvement of CaM-CaMKII-ERK in bisphenol A-induced Sertoli cell apoptosis. Toxicology. 2014; 324:27–34. PMID: 24905940.

49. Sun J, Chi G, Soromou LW, Chen N, Guan M, Wu Q, Wang D, Li H. Preventive effect of imperatorin on acute lung injury induced by lipopolysaccharide in mice. Int Immunopharmacol. 2012; 14:369–374. PMID: 22878138.

50. Tang CH, Yang RS, Chien MY, Chen CC, Fu WM. Enhancement of bone morphogenetic protein-2 expression and bone formation by coumarin derivatives via p38 and ERK-dependent pathway in osteoblasts. Eur J Pharmacol. 2008; 579:40–49. PMID: 17980360.

Fig. 1
Effects of imperatorin on cell viability and PFHxS-increased caspase-3 activity in CGC.
(A) Cells were treated with different concentrations of imperatorin (0~1000 nM) for 24 h. Cell viability was determined by MTS assay. (B) Cells were pretreated with different concentrations of imperatorin (10~1000 nM) or PD98059 (50 µM) for 1 h and treated with either PFHxS (300 µM) or DMSO as a vehicle control for 3 h. Then, cells were incubated in fresh media for 21 h. Caspase-3 activity was measured. Data (fold increase) are mean±SEM of three independent experiments. **p<0.01, ***p<0.001 vs. DMSO. #p<0.05, ###p<0.001 vs. corresponding Control-treated cells. (IPT, imperatorin; PD, PD98059).

Fig. 2
Effects of imperatorin on CGC apoptosis induced by PFHxS.
Cells were pretreated with imperatorin (500 nM), MK801 (1 µM) or AC-DEVE-CHO (10 µM) for 1 h and treated with either PFHxS (300 µM) or DMSO as a vehicle control for 3 h. Then, cells were incubated in fresh media for 21 h. The apoptotic cells were stained with TUNEL (green) and all cells were counterstained with PI (red). (A) TUNEL- and PI-positive cells were monitored by fluorescence microscopy. Representative microscopic images from three independent experiments are presented (magnification, x200). (B). TUNEL– and PI-positive cells were counted. The number of TUNEL-positive cells was expressed as a percentage of the total number of cells. Data (% TUNEL-positive cells) are mean±SEM of three independent experiments. *p<0.05, ***p<0.001 vs. DMSO. ###p&0.001 vs. corresponding Control-treated cells (IPT, imperatorin).
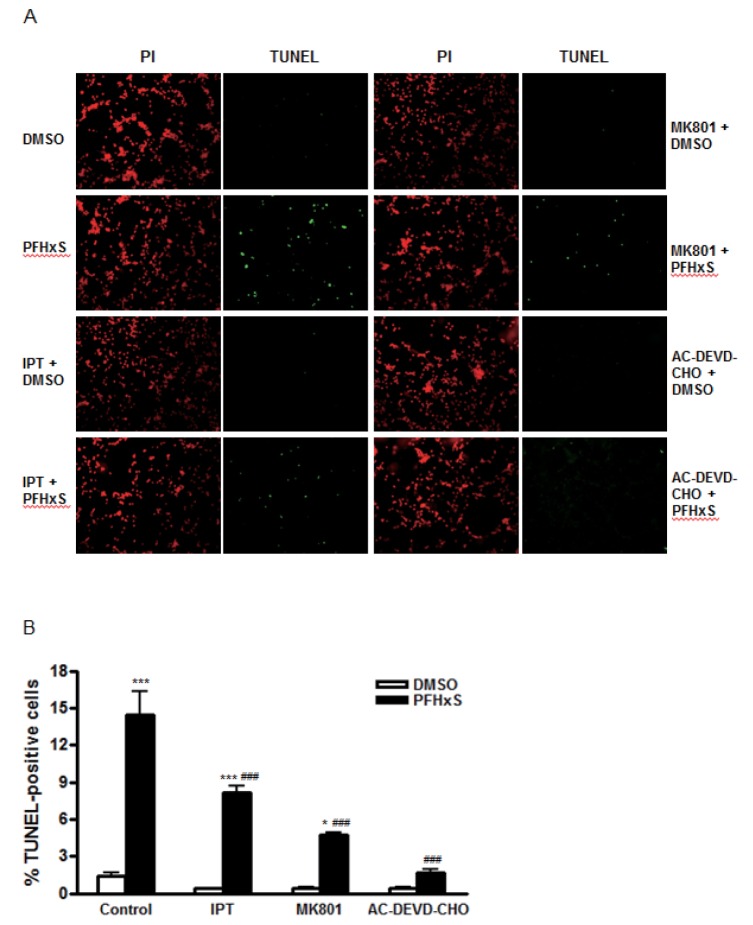
Fig. 3
Effects of imperatorin on PFHxS-induced NMDA receptor activation and Ca2+ influx.
(A) Cells were pretreated with MK801 (1 µM), DTZ (10 µM) or NFD (10 µM) and then stimulated with 300 µM of PFHxS or DMSO as a vehicle control for 3 h. Then, the cells were incubated in fresh media for 21 h to detect caspase-3 activity. (B) Cells were pretreated with imperatorin (100 and 500 nM), MK801 (1 µM), DTZ (10 µM) or NFD (10 µM) and then stimulated with 100 µM NMDA or DMSO as a vehicle control for 15 min. Then, the cells were incubated in fresh media for 24 h to detect caspase-3 activity. (C) Cells were treated with 300 µM PFHxS for different times (0~24 h). The level of intracellular Ca2+ ([Ca2+]i) was measured. (D) Cells were pretreated with imperatorin (100 and 500 nM), MK801 (1 µM), DTZ (10 µM) or NFD (10 µM) and then stimulated with 300 µM of PFHxS for 1 h to detect intracellular [Ca2+]. Data (fold increase) are represented as the mean±SEM of three independent experiments. *p<0.05, **p<0.01, ***p<0.001 vs. DMSO. ###p<0.001 vs. corresponding Control-treated cells (IPT, imperatorin; DTZ, diltiazem; NFD, nifedipine).
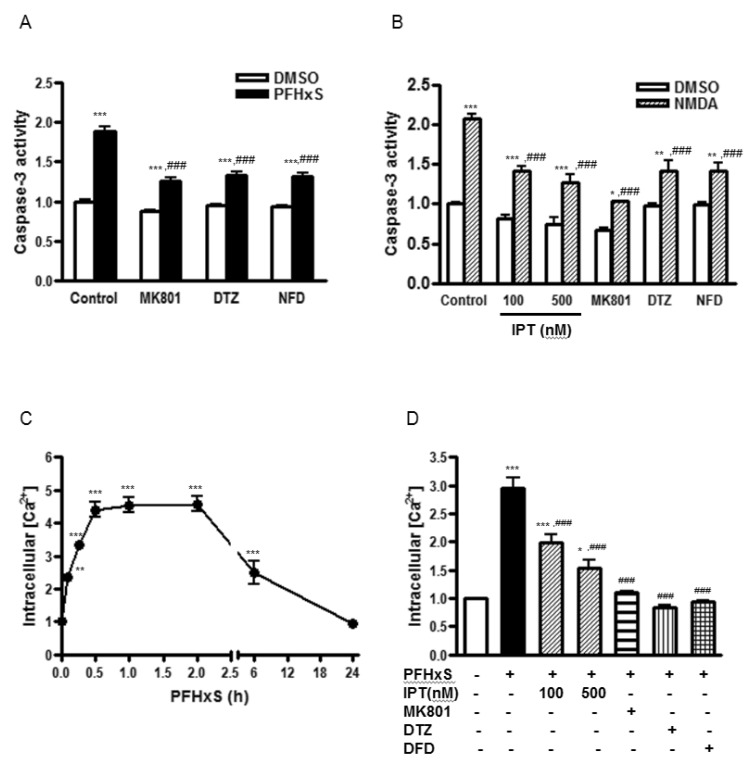
Fig. 4
Effects of imperatorin on PFHxS-induced ERK activation.
Cells were treated with 300 µM PFHxS or DMSO as a vehicle control for 30 min in the presence or absence of (A) imperatorin (50, 100, 500 nM), (B) MK801 (1 µM), DTZ (10 µM), NFD (10 µM) or PD (50 µM). The levels of phosphorylatedand total protein of ERK1/2 were detected by Western blot analysis. The blots were reprobed with GAPDH. The blots represent three independent experiments. The densities of bands were measured and the fold increase in ratio pERK/ERK was presented as mean±SEM of three independent experiments. *p<0.05, **p<0.01, ***p<0.001 vs. DMSO. #p<0.05, ##p<0.01, ###p<0.001 vs. corresponding Control-treated cells (Con, control; IPT, imperatorin; DTZ, diltiazem; NFD, nifedipine; PD, PD98059).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download