INTRODUCTION
METHODS
Materials
Isolation of K49-PLA2
SDS-PAGE and in-gel protein digestion
Identification of proteins by LC-MS/MS
Preparation of human peripheral neutrophils
Lactate dehydrogenase (LDH) activity assay
[Ca2+]i measurement
Statistical analysis
RESULTS
Effect of 'crude' PDE 1 and pure PDE I on [Ca2+]i increase in human neutrophils
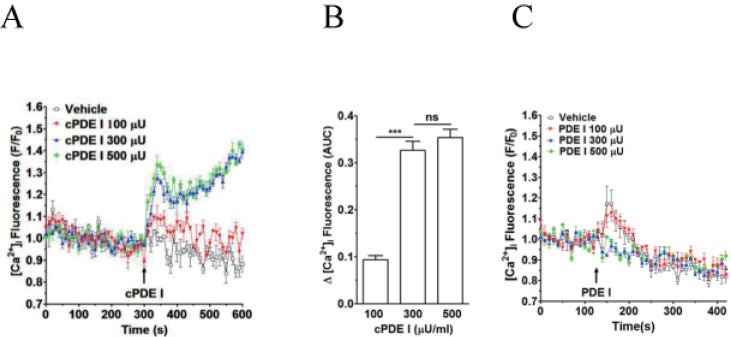 | Fig. 1Effect of crude PDE I (Sigma) and pure PDE I on [Ca2+]i increase in neutrophilsFluo-3 AM-loaded neutrophils were re-suspended in HEPES-PS buffer and plated on 96-well plates at a cell density of 3×106 cells/ml, and then incubated at 37℃ for 10 minutes for cell stabilization. (A) CrudePDE I (100, 300 and 500 µU) was added at 300 s. (B) [Ca2+]i was shown as area under curve (AUC), which was calculated for 300 s (300~600 s). (C) Pure PDE I (100, 300 and 500 µU) was added at 300 s. Changes in [Ca2+]i were expressed as the relative fluorescence intensity of Fluo-3 AM over baseline fluorescence intensity (F/F0). Data points represent the mean±SEM of more than three independent experiments. ***p<0.001.
|
 | Fig. 4Effect of K49-PLA2 on [Ca2+]i in neutrophils.(A) Fluo-3 AM-loaded neutrophils (3×106 cells/ml) were suspended in HEPES buffer. K49-PLA2 (0.1, 0.5, 1, 5 and 10 µg/ml) were added at 300 s. (B) [Ca2+]i was shown as area under curve (AUC), which was calculated for 300 s (300~600 s). (C) Fluo-3 AM-loaded neutrophils (3×106 cells/ml) were suspended in calcium-free HEPES buffer. K49P-LA2 (1 µg/ml) was added at 120 s. Changes in [Ca2+]i were expressed as the relative fluorescence intensity of Fluo-3 AM over baseline fluorescence intensity (F/F0). Data points represent the mean±SEM of more than three independent experiments. ***p<0.001.
|
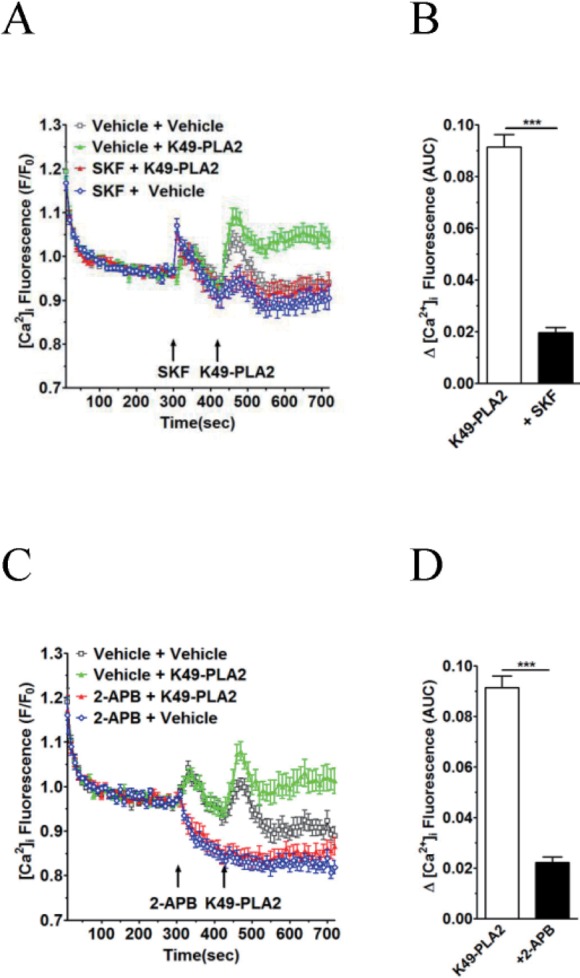 | Fig. 6K49-PLA2-induced [Ca2+]i increase is inhibited by TRP channel inhibitors 2-APB and SKF-96365 in neutrophils.(A and C) Fluo-3 AM-loaded neutrophils (3×106 cells/ml) were suspended in HEPES. 2-APB (30 µM) and SKF-96365 (20 µM) were added for 2 min before the addition of K49-PLA2. (B and D) [Ca2+]i was shown as area under curve (AUC), which was calculated for 300 s (420~720 s). Changes in [Ca2+]i were expressed as the relative fluorescence intensity of Fluo-3 AM over baseline fluorescence intensity (F/F0). Data points represent the mean±SEM of more than three independent experiments. ***p<0.001.
|
Purification of causative factor(s)
 | Fig. 2Isolation of K49-PLA2 from crude PDE I (C. atrox venom) by HPLC.Activity-driven isolation of K49-PLA2 from crudePDE I (C. atrox venom)was done using a three-step chromatography procedure as described in Materials and Methods. (A) Semi-preparative reverse phase C-18 (20×150 mm) column recycling preparative HPLC. (B) Size exclusion HPLC of fraction 10 from semi-preparative reverse phase HPLC. (C) Reverse phase chromatography of the fraction 5 from size exclusion HPLC.
|
Size determination and identification of fraction 2
K49-PLA2 increases concentration-dependently [Ca2+]i in neutrophils via extracellular calcium influx
K49-PLA2 increases [Ca2+]i in human neutrophils without causing membrane disruption
 | Fig. 5K49-PLA2 had no cytotoxic effect on neutrophils.LDH was measured according to LDH measuring kit (Takara) protocol after 1 h-incubation of neutrophils with K49-PLA2 (1 µg/ml). Absorbance was read at 490 nm with fluorescence micro plate reader (SPECTRAmax M2e, Molecular Devices). Medium alone and medium from cells incubated with 0.1% (v/v) of Triton X-100, were used as reference controls for 0% and 100% cytolysis, respectively.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download