Abstract
Here, we investigated whether hyperglycemia and/or free fatty acids (palmitate, PAL) aff ect the expression level of bone morphogenic protein 4 (BMP4), a proatherogenic marker, in endothelial cells and the potential role of BMP4 in diabetic vascular complications. To measure BMP4 expression, human umbilical vein endothelial cells (HUVECs) were exposed to high glucose concentrations and/or PAL for 24 or 72 h, and the effects of these treatments on the expression levels of adhesion molecules and reactive oxygen species (ROS) were examined. BMP4 loss-of-function status was achieved via transfection of a BMP4-specific siRNA. High glucose levels increased BMP4 expression in HUVECs in a dose-dependent manner. PAL potentiated such expression. The levels of adhesion molecules and ROS production increased upon treatment with high glucose and/or PAL, but this eff ect was negated when BMP4 was knocked down via siRNA. Signaling of BMP4, a proinflammatory and pro-atherogenic cytokine marker, was increased by hyperglycemia and PAL. BMP4 induced the expression of infl ammatory adhesion molecules and ROS production. Our work suggests that BMP4 plays a role in atherogenesis induced by high glucose levels and/or PAL.
BMP was originally described as a bone-inducing protein. BMP members of the transforming growth factor β superfamily are cytokines with diverse critical roles in embryonic development, angiogenesis, and cartilage formation [12]. In several tissues, BMP signaling has been linked to regulation of cellular development, pluripotency, differentiation, apoptosis, proliferation, and morphogenesis [13]. BMPs act as proatherogenic mediators in the arterial wall. BMP2, BMP4, and BMP6, and BMP receptors such as Alk1 are upregulated in atheroprone vascular regions and atherosclerotic lesions, indicating that they contribute to plaque formation [34]. Recently, BMPs expressed under hyperglycemic and diabetic conditions have been shown to trigger the overproduction of reactive oxygen species (ROS), which in turn trigger endothelial cell dysfunction and apoptosis [4567].
BMP4 expression is triggered by blood flow disturbances [8]. BMP4 is upregulated in atheroprone regions of blood vessels and may contribute to vascular calcification and the development of atherosclerotic plaques [89]. Exposure to oscillatory shear induces endothelial expression of BMP4, which in turn may activate intercellular adhesion molecule-1 (ICAM-1) expression and monocyte adhesion [89]. BMP4 also acts as a mechanosensitive proinflammatory cytokine that stimulates ROS synthesis by Nox1-dependent NADPH oxidase [710]. In contrast, laminar shear stress and the cAMP/PKA pathway are important negative regulators of BMP4 expression in the vascular endothelium [8]. In vivo, chronic BMP4 infusion into C57BL/6 apolipoprotein E-knockout mice impaired endothelium-dependent vasodilation and induced arterial hypertension in an NADPH oxidase-dependent manner [5].
Chronic exposure of cells (both in vitro and in vivo) to elevated glucose levels (glucotoxicity), fatty acid levels (lipotoxicity), or both (glucolipotoxicity) impairs cellular functions and triggers cell death [11]. In diabetes, exposure of the vascular endothelium to high glucose (HG) levels increases oxidative stress and causes vascular dysfunction [12]. HG also significantly enhances intracellular ROS formation, associated with subsequent apoptosis, in human umbilical vein endothelial cells (HUVECs) [13]. Exposure to HG significantly increases BMP4 expression in HUVECs [14], diabetic mouse aorta, and endothelial cells [15]. Free fatty acids (FFAs), the levels of which are elevated in metabolic syndrome and diabetes, play a crucial role in the pathogenesis of metabolic diseases, including atherosclerosis and type 2 diabetes. Saturated FFAs activate inflammatory signaling pathways in vascular smooth cells, macrophages, and vascular endothelial cells [16] and are lipotoxic to endothelial cells, directly inducing endothelial dysfunction and/or apoptosis by increasing oxidative stress [12]. Serum BMP4 levels are inversely correlated with those of triglycerides and FFAs, as well as with arterial stiffness and carotid atherosclerosis in patients with type 2 diabetes [17]. Although diabetes triggers endothelial dysfunction, in turn promoting diabetic vascular disease, the associations between BMPs and vascular disease induced by hyperglycemia or high-level FFAs remain incompletely understood.
Here, we investigated whether hyperglycemia and/or PAL affect BMP4 expression in endothelial cells, and how BMP4 might play a role in the vascular complications of diabetes. We investigated whether BMP4 is associated with endothelial dysfunction caused by altered levels of adhesion molecules and ROS production triggered by hyperglycemia and PAL.
Primary HUVECs (Modern Cell&Tissue Technologies, Seoul, Korea) were cultured in the endothelial cell basal medium provided in the EBM-2 BulletKit (Lonza, Basel, Switzerland), supplemented with 20% FBS. Cells were grown to confluence at 37℃ in a humidified atmosphere of 5% CO2/95% air. Cells were used in the experiments after passage 4.
HUVECs were plated in 60-mm culture dishes at a density of 1×106/dish. After 16~24 h, the cells were washed with DMEM containing 2% FBS, antibiotics, and antimycotics but no growth factors and then incubated in the presence of HG (D-glucose 500 mg/dl), palmitate (PAL; 500 µM, Sigma-Aldrich, St. Louis, MO, USA), or both HG+PAL for 24~72 h. The fatty acids were dissolved in ethanol (40 mM solution) prior to their addition to the medium. Control cells were incubated in the presence of 0.25% vehicle (ethanol).
Cells were seeded into 6-well plates at a density of 2×105/well with growth media. The amount of siRNA was optimized per the manufacturer's instructions. Predesigned siRNAs targeting BMP4 (three Silence® siRNAs from Ambion and one from Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a scrambled siRNA containing the same nucleotide content were evaluated. When compared with unrelated control siRNA and scrambled siRNA, the specific siRNAs resulted in an 80~90% decrease in mRNA and protein levels, as determined by RT-PCR and Western blotting, respectively. The siRNA that provided the most effective inhibition (>60%) was used for the experiments. BMP4 siRNA transfection was performed according to the manufacturer's protocol (Santa Cruz Biotechnology). At 6 h post-transfection, the media were refreshed, and the cells were treated with HG, PAL, or HG+PAL for 72 h. Media were changed daily.
Cells were lysed in ice-cold lysis buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 2 mM EDTA, 1% Nonidet P-40, 10 mM NaF, 1 mM Na3VO4, 10 mM sodium pyrophosphate, 1 mM phenylmethylsulfonyl fluoride, 10 mg/ml aprotinin, 10 mg/ml leupeptin, 0.1 mg/ml soybean inhibitor). Cell lysates were centrifuged at 15,000 rpm for 10 min at 4℃. Protein concentrations were measured by the BCA method using BSA as the standard. Proteins (30 µg) were separated by 8~10% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked with 5% fat-free milk for 1 h in Tris-buffered saline (TBS; 25 mM Tris-HCl, pH 7.6, and 150 mM NaCl) containing 0.1% Tween 20 (TBS-T) and then incubated with the following primary rabbit or mouse antibodies: anti-BMP4, -ICAM-1, -VCAM-1 and -E-selectin (all from Santa Cruz Biotechnology), and anti-β-actin (Sigma-Aldrich). The antibodies were diluted 1:500~1:2,000 in 1% fat-free milk in TBS-T and incubated at 4℃ overnight. After washing, the membranes were incubated with secondary peroxidase-conjugated anti-mouse and anti-rabbit antibodies (Bio-Rad Laboratories, Hercules, CA, USA) diluted 1:1,000 in 1% fat-free milk in TBS-T at room temperature for 1 h. Detection was achieved using Pierce ECL Plus Western Blotting Substrate (ThermoFisher Scientific Inc., Rockford, IL, USA).
We measured the adhesion of fluorescently labeled human monocyte cells (THP-1, Korea Cell line Bank, Seoul, Korea) onto confluent HUVEC monolayers. Cells were grown to confluence in 6-well plates and, after transfection with BMP4 siRNA, were treated with HG, FFA and HG+FFA for 72 h at 37℃. TNF-α was used as a positive control. THP-1 cells were labeled with the fluorescent dye BCECF-AM (5 µmol/l final concentration, Molecular Probes Inc., Rockford, IL, USA) in serum-free RPMI medium for 30 min at 37℃. Cells were then washed twice with pre-warmed (37℃) RPMI medium. Fluorescently labeled THP-1 cells (1×106/well) were added to confluent HUVECs for 30 min at 37℃. Non-adherent THP-1 cells were removed by careful washing (three times) with pre-warmed RPMI medium. Cells were fixed with 1 ml 4% paraformaldehyde for 5 min. PBS was added to each well, and fluorescence was then measured using a multilabel counter system (Perkin Elmer, Waltham, MA, USA; excitation: 485 nm; emission: 528 nm). Controls included the measurement of total fluorescence from labeled cells before adhesion, the measurement of autofluorescence from unlabeled cells, and the measurement of monocyte adhesion to HUVEC-free microplate wells.
Intracellular ROS formation in HUVECs was detected using the f luorescent probe 5-(and 6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA, Molecular Probes Inc.) according to the manufacturer's protocol. Fluorescence was then measured using a multilabel counter system (Perkin Elmer, excitation: 485 nm; emission: 528 nm). Preliminarily, we confirmed the satisfactory efficacy of this probe in detecting intracellular ROS signals induced by H2O2 in HUVECs.
Data are expressed as means±SEM. Differences between groups were evaluated using SPSS 13.0 (Chicago, IL, USA). The independent t-test, one-way ANOVA, and Kruskal-Wallis test were used to analyze the quantitative variables between groups. A p value<0.05 was deemed to indicate significance.
The cells were washed with DMEM (containing 2% FBS, without growth factors) 16~24 h after plating and then incubated with glucose (D-glucose 100, 500, 1000 mg/dL) and mannitol (25 mmol) in DMEM for 24 h. The BMP4 levels in HUVECs after exposure to glucose are shown in Fig. 1. BMP4 production was minimal after incubation with 100 mg/dL glucose and maximal after incubation with 1,000 mg/dL glucose (p<0.05, Fig. 1). Although BMP4 expression was increased somewhat after incubation with 500 mg/dL glucose compared with 100 mg/dL glucose, the difference was not significant (p=NS). Nevertheless, HUVECs cultured in 100 mg/dL plus 25 mmol mannitol showed no increase in BMP4 expression, indicating that high osmolarity did not affect the expression of BMP4 (100 mg/dL glucose with vs. without 25 mmol mannitol, p=NS; Fig. 1).
HUVECs were incubated with HG (500 mg/dL), PAL (500 µM), and HG+PAL in DMEM media (containing 2% FBS, without growth factors) for 24 or 72 h. PAL treatment increased BMP4 levels in HUVECs to levels significantly higher than those attained upon exposure to HG alone after 1 day (p<0.05, Fig. 2). Although BMP4 expression was somewhat reduced with HG + PAL, it was increased significantly compared with incubation in 100 or 500 mg/dL glucose after 1 day. As shown in Fig. 2, the BMP4 protein level in HUVECs was increased 3.2-fold from 1 to 3 days of exposure to HG+PAL (p<0.05).
We used siRNA techniques to inhibit BMP4-dependent pathways selectively and examine the effects of HG, PAL, and HG+PAL incubation on the expression of ICAM-1, VCAM-1, and E-selectin. The protein levels of these adhesion molecules in HUVECs incubated with HG alone, PAL alone, or HG+PAL (in DMEM containing 2% FBS, without growth factors) for 72 h were explored by Western blotting. Compared with unstimulated cells, ICAM-1, VCAM-1, and E-selectin protein levels were significantly increased (p<0.05, Fig. 3). The knockdown of BMP4 using specific siRNAs (scrambled siRNA served as the control) prevented these increases in VCAM-1 and E-selectin levels under all three conditions (p<0.05, Fig. 3). Unexpectedly, ICAM-1 expression was not inhibited by sRNA-mediated BMP4 knockdown and increased further upon exposure to HG+PAL (Fig. 3). Western blotting confirmed that the BMP4 protein level was reduced by more than 60% by siRNA.
We examined the effects of HG, PAL, and HG+PAL incubation (in DMEM containing 2% FBS, without growth factors) for 72 h in a monocyte adhesion assay. Treatment with PAL significantly increased monocyte adherence to cultured HUVECs, and transfection of BMP4 siRNAs inhibited this process (p<0.05, Fig. 4). PAL alone or HG+PAL significantly increased monocyte adhesion (p<0.05), while depletion of BMP4 abolished PAL-induced monocyte adhesion (p<0.05, Fig. 4).
We examined the effects of HG, PAL, and HG+PAL incubation (in DMEM containing 2% FBS, without growth factors) for 72 h on the production of ROS. After siRNA-mediated BMP4 knockdown, ROS production was not elevated in HUVECs exposed to HG alone, PAL alone, or HG+PAL; however, ROS production was elevated under BMP4 expression (p<0.05, Fig. 5).
Postprandial increases in lipid levels and hyperglycemia induce oxidative stress, with implications for the pathogenesis of cardiovascular diseases and diabetic complications. Under diabetic conditions, long-term hyperglycemia and high circulating FFA levels cause significant endothelial damage [1819]. We demonstrated that BMP4 induced by hyperglycemia and high PAL levels acts as a proatherogenic cytokine in HUVECs.
BMPR1A and BMPR2 are involved in BMP4 signal transduction, and the binding of BMP4 to BMPR2 triggers the recruitment and phosphorylation of BMPRIA [2021]. Thus, different BMP family members stimulate or inhibit endothelial cell angiogenesis, proliferation, and migration. Our present work may also suggest a role for BMP2, similar to that of BMP4, in HUVECs under HG conditions [22]. Although BMP2 and BMP4 exhibit a high level of sequence similarity and likely act on the same receptor, the biological roles of the two cytokines, in fact, may differ [372324]. Kim et al. [24] reported that the expression levels of BMP2 and BMP4 were increased in human atherosclerotic plaques and coronary artery endothelium overlying atherosclerotic lesions. BMP2 and BMP4 exert proinflammatory, pro-oxidant, and pro-hypertensive effects on systemic arteries [10]. BMP2 and BMP4 may play distinct roles in the vascular atherogenesis associated with hyperglycemia; BMP4 may induce mainly pro-angiogenic effects and BMP2 pro-calcific effects [37]. BMP4 is preferentially expressed in endothelial cells, and BMP2 is both expressed in the endothelium and secreted into the growth medium [723].
The BMP4 expression levels in type 2 diabetics exhibiting venous endothelial dysfunction [14] and in HUVECs treated with HG were affected in a dose-dependent manner (p<0.05, Fig. 1). Zhang et al. [15] found that HG increased BMP4 expression in the aorta of C57BL6 mice and mouse aortic endothelial cells. Previously, we found that serum BMP4 levels were associated with human adiposity and metabolic syndrome [17]. BMP4 expression levels in HUVECs were increased after chronic exposure to PAL compared with HG alone (Fig. 2). BMP2 and BMP4 produced by FFA-stimulated macrophages play roles in vascular smooth muscle cell proliferation, migration, and phenotypic changes [25]. PAL increased BMP4 production in human endothelial cells, and a high-fat diet increased BMP4 levels in thoracic aortic tissue of C57BL/6 mice [26]. Therefore, BMP4 is associated with atherosclerosis induced by either PAL or hyperglycemia.
The expression levels of endothelial adhesion molecules and proteins associated with inflammation and cellular stress show strong correlations with increased BMP activity [8]. However, BMP4 siRNA did not significantly reduce ICAM-1 expression, even though VCAM-1 and E-selectin levels were reduced effectively (Fig. 3). ICAM-1-enhanced monocyte recruitment is a potential mode of growth for atherosclerotic plaques [6]. Therefore, to enhance the treatment of vascular inflammation, it is important to understand how ICAM-1 expression on the endothelial cell surface is regulated and to identify regulators of ICAM-1 expression [6]. Although both VCAM-1 and ICAM-1 are upregulated in atherosclerotic lesions, VCAM-1, but not ICAM-1, is associated with early-stage atherosclerosis [27]. Jo et al. [8] reported that staining of ICAM-1 in human coronary arteries also resulted in staining of BMP4, and the extent of the (mutual) staining was increased as the endothelial patches expanded. We found that the combination of HG and PAL significantly increased the levels of BMP4 and ICAM-1 (Fig. 2 and 3). In addition, the hyperglycemia- and/or PAL-induced expression of these adhesion molecules increased THP-1-mediated cell adhesion (Fig. 4). Thus, BMP4 inhibition is likely to exert strong anti-inflammatory effects on vascular cells, and the increase in ICAM-1 caused by chronic exposure to HG and PAL may be associated with late-stage atherosclerosis.
ROS are critically involved in signal transduction and physiological regulation of vascular function. Under pathological conditions, increased ROS bioavailability (oxidative stress) triggers signaling events that promote endothelial dysfunction, vascular inflammation, and arterial remodeling [1928]. Activation of BMP4 signaling triggers both Smad-dependent and -independent pathways, including the activation of NADPH oxidase, thereby elevating ROS production [15]. Csiszar et al. [10] showed that atherosclerosis involving pulmonary vascular endothelial cells may be attributed to a lack of BMP4-induced endothelial activation; such cells exhibited superior resistance to the pro-oxidant effects of BMP4. Du et al. [16] showed that high levels of PAL and glucose increased endothelial ROS production in the retinal and aortic endothelium of high-fat diet-fed ZDF rats. Zhang et al. [15] found that the levels of superoxide anions in C57BL/6 and dbb/dbb mouse aortas were significantly increased after 48 h of exposure to HG levels, and treatment with BMP4 siRNA reversed these effects. The increases in vascular cell ROS production caused by HG and/or PAL may alter vascular function, partially explaining the rapid acceleration of atherosclerosis in patients with metabolic syndrome.
In summary, we showed BMP4-induced endothelial dysfunction in HUVECs, providing the first evidence that HG and FFA (palmitate) combination treatment stimulate ROS production and adhesion molecules via BMP4 expression in endothelial cells. BMP4, as a proinflammatory and proatherogenic cytokine, represents the earliest measurable marker of atherosclerosis. Our findings afford further insight into the mechanisms by which BMP4 induces atherosclerosis in diabetic patients.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2012R1A1A2007098).
Notes
Author contributions: O.K.H. and H.S.K. participated in the design of the study. O.K.H. and H.S.K. performed the experiments. O.K.H., S.J.Y., J.W.S., M.K.K., K.H.B., K.H.S., B.Y.C., H.J. and H.S.K. conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
1. Wong WT, Tian XY, Chen Y, Leung FP, Liu L, Lee HK, Ng CF, Xu A, Yao X, Vanhoutte PM, Tipoe GL, Huang Y. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: implications on hypertension. Circ Res. 2010; 107:984–991. PMID: 20724703.
2. Cai J, Pardali E, Sánchez-Duffhues G, ten Dijke P. BMP signaling in vascular diseases. FEBS Lett. 2012; 586:1993–2002. PMID: 22710160.

3. Maciel TT, Kempf H, Campos AH. Targeting bone morphogenetic protein signaling on renal and vascular diseases. Curr Opin Nephrol Hypertens. 2010; 19:26–31. PMID: 19823085.

4. Boström KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res. 2011; 108:446–457. PMID: 21193740.

5. Csiszar A, Labinskyy N, Smith KE, Rivera A, Bakker EN, Jo H, Gardner J, Orosz Z, Ungvari Z. Downregulation of bone morphogenetic protein 4 expression in coronary arterial endothelial cells: role of shear stress and the cAMP/protein kinase A pathway. Arterioscler Thromb Vasc Biol. 2007; 27:776–782. PMID: 17272757.
6. Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassègue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004; 95:773–779. PMID: 15388638.

7. Koga M, Engberding N, Dikalova AE, Chang KH, Seidel-Rogol B, Long JS, Lassègue B, Jo H, Griendling KK. The bone morphogenic protein inhibitor, noggin, reduces glycemia and vascular inflammation in db/db mice. Am J Physiol Heart Circ Physiol. 2013; 305:H747–H755. PMID: 23812391.

8. Jo H, Song H, Mowbray A. Role of NADPH oxidases in disturbed flow- and BMP4-induced inflammation and atherosclerosis. Antioxid Redox Signal. 2006; 8:1609–1619. PMID: 16987015.
9. Csiszar A, Lehoux S, Ungvari Z. Hemodynamic forces, vascular oxidative stress, and regulation of BMP-2/4 expression. Antioxid Redox Signal. 2009; 11:1683–1697. PMID: 19320562.

10. Csiszar A, Labinskyy N, Jo H, Ballabh P, Ungvari Z. Differential proinflammatory and prooxidant effects of bone morphogenetic protein-4 in coronary and pulmonary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2008; 295:H569–H577. PMID: 18539760.

11. Jaimes EA, Hua P, Tian RX, Raij L. Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am J Physiol Renal Physiol. 2010; 298:F125–F132. PMID: 19864304.

12. Chinen I, Shimabukuro M, Yamakawa K, Higa N, Matsuzaki T, Noguchi K, Ueda S, Sakanashi M, Takasu N. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology. 2007; 148:160–165. PMID: 17023526.

13. Hou Q, Lei M, Hu K, Wang M. The effects of high glucose levels on reactive oxygen species-induced apoptosis and involved signaling in human vascular endothelial cells. Cardiovasc Toxicol. 2015; 15:140–146. PMID: 25158671.

14. Hu J, Liu J, Kwok MW, Wong RH, Huang Y, Wan S. Bone morphogenic protein-4 contributes to venous endothelial dysfunction in patients with diabetes undergoing coronary revascularization. Ann Thorac Surg. 2013; 95:1331–1339. PMID: 23522199.

15. Zhang Y, Liu J, Tian XY, Wong WT, Chen Y, Wang L, Luo J, Cheang WS, Lau CW, Kwan KM, Wang N, Yao X, Huang Y. Inhibition of bone morphogenic protein 4 restores endothelial function in db/db diabetic mice. Arterioscler Thromb Vasc Biol. 2014; 34:152–159. PMID: 24202302.
16. Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006; 116:1071–1080. PMID: 16528409.

17. Son JW, Jang EH, Kim MK, Baek KH, Song KH, Yoon KH, Cha BY, Son HY, Lee KW, Jo H, Kwon HS. Serum BMP-4 levels in relation to arterial stiffness and carotid atherosclerosis in patients with Type 2 diabetes. Biomark Med. 2011; 5:827–835. PMID: 22103619.

18. Zhu P, Chen G, You T, Yao J, Jiang Q, Lin X, Shen X, Qiao Y, Lin L. High FFA-induced proliferation and apoptosis in human umbilical vein endothelial cell partly through Wnt/beta-catenin signal pathway. Mol Cell Biochem. 2010; 338:123–131. PMID: 19967550.
19. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000; 49:1939–1945. PMID: 11078463.

20. Upton PD, Long L, Trembath RC, Morrell NW. Functional characterization of bone morphogenetic protein binding sites and Smad1/5 activation in human vascular cells. Mol Pharmacol. 2008; 73:539–552. PMID: 17989347.

21. Wu J, Yu Z, Su D. BMP4 protects rat pulmonary arterial smooth muscle cells from apoptosis by PI3K/AKT/Smad1/5/8 signaling. Int J Mol Sci. 2014; 15:13738–13754. PMID: 25110865.

22. Zhang M, Zhou SH, Zhao S, Li XP, Liu LP, Shen XQ. Pioglitazone can downregulate bone morphogenetic protein-2 expression induced by high glucose in human umbilical vein endothelial cells. Pharmacology. 2008; 81:312–316. PMID: 18311072.

23. Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006; 168:629–638. PMID: 16436676.

24. Kim CW, Song H, Kumar S, Nam D, Kwon HS, Chang KH, Son DJ, Kang DW, Brodie SA, Weiss D, Vega JD, Alberts-Grill N, Griendling K, Taylor WR, Jo H. Anti-inflammatory and antiatherogenic role of BMP receptor II in endothelial cells. Arterioscler Thromb Vasc Biol. 2013; 33:1350–1359. PMID: 23559633.

25. Chung JH, Jeon HJ, Hong SY, Lee da L, Lee KH, Kim SH, Han YS, Manabe I, Miller YI, Lee SH. Palmitate promotes the paracrine effects of macrophages on vascular smooth muscle cells: the role of bone morphogenetic proteins. PLoS One. 2012; 7:e29100. PMID: 22363399.

26. Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, Handa P, Schwartz MW, Kim F. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol. 2009; 29:1370–1375. PMID: 19542021.
27. Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001; 107:1255–1262. PMID: 11375415.

28. Xu S, He Y, Vokurkova M, Touyz RM. Endothelial cells negatively modulate reactive oxygen species generation in vascular smooth muscle cells: role of thioredoxin. Hypertension. 2009; 54:427–433. PMID: 19564543.
Fig. 1
Glucose increased expression of BMP4 in dose-dependent manners.
After 16~24 h plating, the cells were washed with DMEM media (containing 2% FBS, w/o growth factor), and then incubated with glucose (D-glucose 100, 500, 1000 mg/dL), and mannitol (D-glucose 100 mg/dL+Mannitol 25 mmol ) for 24 h. High glucose increase BMP4 expression in dose-dependent manner in HUVECs. Data are expressed as the mean±SEM of four independent observations in separate cell culture wells. *p<0.05 vs. 100 mg/dL glucose.
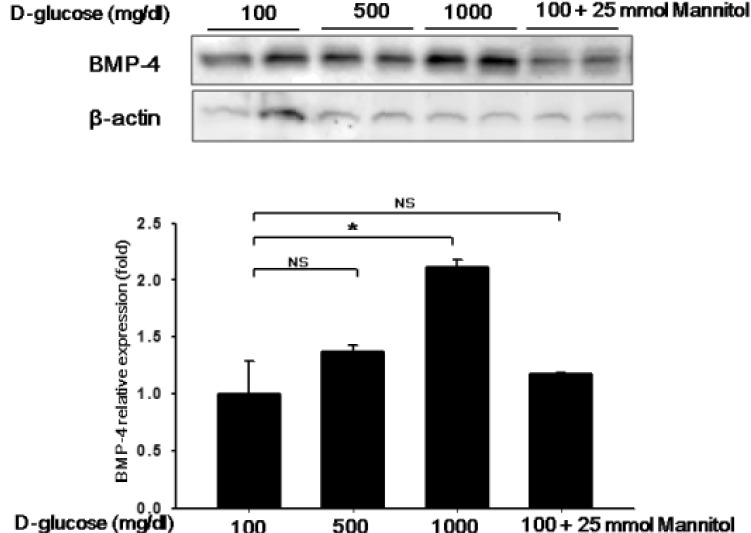
Fig. 2
High glucose and FFA increased expression of BMP4 in time-dependent manners.
HUVECs incubated with HG, PAL, and HG/PAL combination with DMEM media (containing 2% FBS, w/o growth factor) for 24 h or 72 h. BMP4 increased by high glucose, and this increase in PAL accelerates more in time-dependent manner. Data are expressed as the mean±SEM of four independent observations in separate cell culture wells. *p<0.05 vs. NG, NG; 100 mg/dL glucose, HG; 500 mg/dL glucose, PAL; 500 µM palmitate.
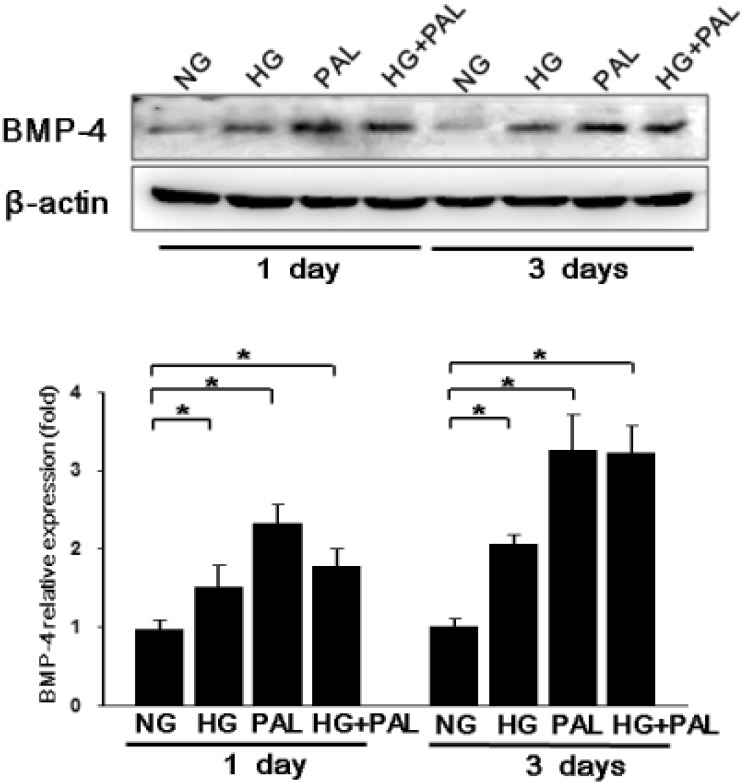
Fig. 3
Activation of BMP4 induced by high glucose and FFA ef fects on various adhesion molecues.
We used siRNA techniques to selectively deplete BMP4-dependent pathways and examine the effect of HG, PAL, HG/PAL with DMEM media (containing 2% FBS, w/o growth factor) for 72 h. Expression of adhesion molecules were increased by treatments with HG and/or PAL. When BMP4 was knockdowned by siRNA, this expression was blunted. Data are expressed as the mean±SEM of four independent observations in separate cell culture wells. *p<0.05, NG; 100mg/dL glucose, HG; 500 mg/dL glucose, PAL; 500 µM palmitate.

Fig. 4
High glucose and FFA induced BMP4 activation increase monocyte adhesion.
We used siRNA techniques to selectively deplete BMP4-dependent pathways and examine the effect of HG, PAL, HG/PAL with DMEM media (containing 2% FBS, w/o growth factor) for 72 h. In monocyte adhesion assay, HG with PAL induced monocyte adhesion in HUVECs and it was prevented by BMP4 siRNA. Data are expressed as the mean±SEM of four independent observations in separate cell culture wells. *p<0.05 vs. NG, NG; 100 mg/dL glucose, HG; 500 mg/dL glucose, PAL; 500 µM palmitate.

Fig. 5
High glucose and FFA induced BMP4 activation increases ROS production.
We used siRNA techniques to selectively deplete BMP4-dependent pathways and examine the effect of HG, PAL, HG/PAL with DMEM media (containing 2% FBS, w/o growth factor) for 72 h. HG and/or PAL increases ROS production in HUVECs, BMP4 knockdown ameliorated this response. Data are expressed as the mean±SEM of four independent observations in separate cell culture wells. *p<0.05, NG; 100 mg/dL glucose, HG; 500 mg/dL glucose, PAL; 500 µM FFA.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download