Abstract
Present study aimed to investigate the eff ect of curcumin-pretreatment on intestinal I/R injury and on intestinal mucosa barrier. Thirty Wistar rats were randomly divided into: sham, I/R, and curcumin groups (n=10). Animals in curcumin group were pretreated with curcumin by gastric gavage (200 mg/kg) for 2 days before I/R. Small intestine tissues were prepared for Haematoxylin & Eosin (H&E) staining. Serum diamine oxidase (DAO) and tumor necrosis factor (TNF)-α levels were measured. Expression of intestinal TNF-α and tight junction protein (ZO-1) proteins was detected by Western blot and/or immunohistochemistry. Serum DAO level and serum and intestinal TNF-α leves were signifi cantly increased after I/R, and the values were markedly reduced by curcumin pretreatment although still higher than that of sham group (p<0.05 or p<0.001). H&E staining showed the significant injury to intestinal mucosa following I/R, and curcumin pretreatment signifi cantly improved the histological structure of intestinal mucosa. I/R insult also induced significantly down-regulated expression of ZO-1, and the eff ect was dramatically attenuated by curcumin-pretreatment. Curcumin may protect the intestine from I/R injury through restoration of the epithelial structure, promotion of the recovery of intestinal permeability, as well as enhancement of ZO-1 protein expression, and this eff ect may be partly attributed to the TNF-α related pathway.
Intestinal ischemia-reperfusion (I/R) is a potentially life-threatening complication of the digestive organ transplantation and other abdominal surgeries that delays the recovery of patient and leads to multiple organ failure [12]. Intestinal I/R can cause the histological evidence of mucosal injury and gut dysfunction characterized by increased intestinal epithelial permeability and impaired motility [3].
As the principal structures responsible for restricting the paracellular movement of compounds across the intestinal mucosa, tight junctions (TJs) compose of a cluster of cytoplasmic proteins and function as occluding barriers by maintaining cellular polarity and homeostasis and regulating the permeability of paracellular spaces in the epithelium [4]. The altered distribution of TJ proteins that associated with functional TJ deficiencies was demonstrated after intestinal I/R injury in an animal model [5]. Zonula occludens protein-1 (ZO-1), a member of the membrane-associated guanylate kinase family of proteins, is the first characterized TJ protein that acts as a scaffold for organization of transmembrane TJ proteins and recruits various signaling molecules to TJs [6]. The impaired intestinal barrier function often results from the changes in TJ protein expression, therefore such a mechanism may also be assumed for I/R injury. Recent study by Shen et al. indicated that I/R insult resulted in the disruption of TJs and an increase in intestinal permeability, along with the downregulated expression of ZO-1 protein [7]. The loss of ZO-1 and increased permeability may in turn precede the development of significant intestinal inflammation [8].
Curcumin (diferuloylmethane, 1,7-Bis (4-hydroxy-3-methoxyphenyl)-1,6 heptadiene-3, 5-di-one, C21H20O6), commonly known as turmeric, is the yellow bioactive ingredient derived from the powdered dry rhizomes of Curcuma longa Linn (Family Zingiberaceae). It has been extensively evaluated as a therapeutic molecule for a broad range of common ailments, such as cancer, diabetes, cardiovascular disorders, and against various infectious diseases [91011]. A number of studies have reported protective effects of this polyphenol in intestinal I/R injury [121314]. Although several molecular mechanisms have been proposed for curcumin benefits against ischemic injury [15], the potential mechanism is still unclear. None of the previous studies, to our knowledge, have evaluated the effects of curcumin on intestinal permeability and ZO-1 protein expression following I/R insult. The present study was designed to investigate the protective effect of curcumin on intestinal I/R injury, with an emphasis on the mechanisms of intestinal permeability and ZO-1 expression.
Male specific pathogen-free (SPF) Wistar rats, weighing between 180 and 200 g, were purchased from people's liberation army military academy of medical sciences animal experiment center, China. Thirty rats were randomly divided into 3 groups: group I (sham group; n=10), rats underwent laparotomy; group II (I/R group; n=10), occlusion of the superior mesenteric artery (SMA) for 45 minutes followed by 24 h of reperfusion; and group III, (curcumin group; n=10), animals were pretreated with curcumin (Sigma, St Louis, MO, USA) by gastric gavage (200 mg/kg) 2 days before I/R insult. All protocols were approved by the Institutional Animal Care and Use Committee and were in compliance with the Guidelines for the Care and Use of Laboratory Animals.
After overnight fasting, all animals were anesthetized by intraperitoneal injection of 5% chloral hydrate and subjected to surgical procedures as described previously [12]. Briefly, after abdomen opened through a midline incision under sterile conditions, the superior mesenteric artery (SMA) was exposed and occluded using a small atraumatic clamp. Subsequent to the ischemic period of 45 min, the microvascular clamp was removed and reperfusion started for 24 h. At the end of the experiments, animals were sacrificed for hematologic and biochemical analyses.
From each rat, portal venous blood was collected and analyzed for serum diamine oxidase (DAO) and TNF-α levels using an ELISA kit (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions.
Ileal tissue was obtained for determinations of tissue ZO-1 levels and for histopathologic examination. Ileal segment was fixed in 10% formaldehyde for histopathologic examination, and de-paraffinized sections (3 µm in thickness) were stained with hematoxylin and eosin (H&E) and examined using light microscopy to confirm intestinal damage after I/R. The degree of intestinal tissue injury was evaluated as described previously [16]. The serial sections to those above were used for immunohistochemistry, and rehydrated before immunostaining. After blocking, the sections were incubated with rabbit anti-rat ZO-1 antibody (Invitrogen, Eugene, OR, USA). Negative controls were performed by exchange of primary antibody for PBS.
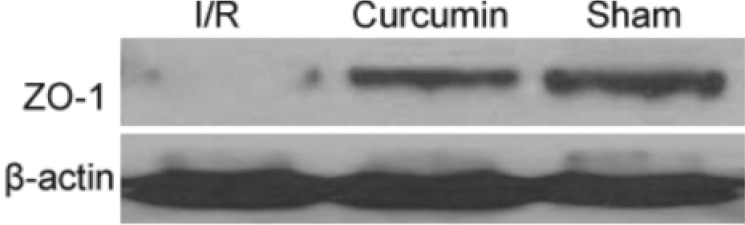
Western blot was further performed to determine the protein expressions of ZO-1 and TNF-α as previously described [8]. Protein extracts from ileums were analyzed by Western blot with rabbit anti-rat ZO-1 antibody (Invitrogen, Eugene, OR, USA) and anti-TNF-α antibody (Abcam, Cambridge, UK), respectively. Equal loading was confirmed with an anti-β-actin antibody.
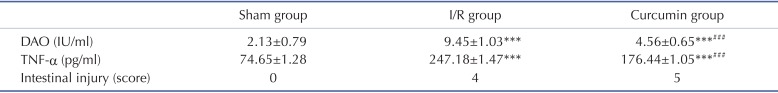
H&E staining showed normal histomorphology of the small intestine with no epithelial disruption in the sham operated rats (Fig. 1A), and intestinal I/R insult resulted in significant injury to intestinal mucosa. The typical histologic features occurred in I/R group were characterized by massive destruction of the villi, multiple erosions, inflammatory cell infiltration, necrosis and hemorrhage of the intestinal wall (Fig. 1B). Curcumin pretreatment significantly improved the histological structure of the intestinal mucosa (Fig. 1C). The scores of intestinal tissue injury were 4 in I/R group and 1 in curcumin group (Table 1).
The serum levels of DAO were significantly increased in I/R and curcumin-pretreated I/R injury rats, when compared with the Sham operated ones. These confirmed that I/R injury increased intestinal permeability. Meanwhile, compared to I/R group, pretreatment with curcumin significantly reduced the serum DAO levels of I/R injury rats (Table 1; p<0.001).
Compared to the sham group, the serum TNF-α level increased significantly in I/R injury group. Whereas, the serum level of TNF-α in curcumin group was markedly down-regulated by curcumin administration, but the value remained higher than that in the Sham group (Table 1; p<0.001). The consistent results obtained by Western blot analysis showed the significantly enhanced expression of intestinal TNF-α following the I/R insult (p<0.001, Fig. 2), this effect was partially reversed by curcumin pretreatment (p<0.05), although the intestinal TNF-α level was still significantly higher in curcumin group than in the shamp group (p<0.001).
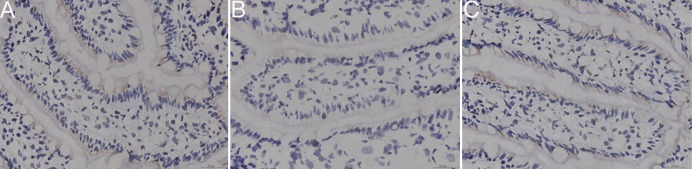
Immunohistochemistry analysis showed obvious changes in the localization and expression of ZO-1 protein following intestinal I/R on ischemic intestinal tissue. In general, loss of continuity in the distribution and attenuation of ZO-1 expression was found following I/R (Fig. 3). There was a significant decrease in the mean optical density values of ZO-1 in I/R group when compared with sham group (p<0.01). I/R induced attenuation in immunoreactivity for ZO-1 was significantly reversed by pretreatment I/R insulted rats with curcumin, although the value was still significantly higher than that in the sham operated rats.
To confirm the effect of focal I/R on ZO-1, we detected the protein expression levels of ZO-1 by Western blot. As shown in Fig. 4, Western blot results were consistent with the findings obtained by immunohistochemistry analysis. Accordingly, the expressions of ZO-1 was significantly attenuated by I/R insult (p<0.01), and this effect was compromised to some extent by curcumin administration (p<0.01).
Small intestine is composed of labile cells that make it particularly sensitive to I/R insult, and I/R injury to the gut is a common event in a variety of clinical conditions [17]. Intestinal I/R can cause damage to epithelial cells, disrupt mucosal integrity and small intestine function, followed by the increased mucosal and vascular permeability, systemic inflammation, as well as multiple organ dysfunction and even death [18]. No effective treatment is available for intestinal I/R injury up to now. A protective effect of curcumin against intestinal I/R injury has been demonstrated by the mechanism related to its well-known antioxidant and other protective properties [12131415]. However, studies of the effect of curcumin pretreatment on the intestinal barrier function are scarce. In the present study, the therapeutic potential of curcumin was evaluated in an experimental rat model of intestinal I/R injury. Our study indicated that intestinal I/R resulted in the histopathological damage and increased intestinal permeability in response to SMA occlusion. Downregulated tissue expression of ZO-1 protein observed further supports the hypothesis that intestinal I/R resulted in intestinal barrier dysfunction with pronounced TJs disruption. Our data were consistent with these findings [7]. This study also revealed that pretreatment with curcumin can effectively ameliorate the intestinal permeability and pathological damage associated with I/R injury, demonstrating a protective effect against intestinal damage resulted from intestinal I/R. This is the first report to show that curcumin can prevent an intestinal barrier dysfunction induced by I/R.
The intestinal epithelial barrier is an essential defense mechanism against intestinal penetration of luminal bacteria and antigens, and the integrity of the structure is generally maintained by TJs [19]. Defective intestinal epithelial TJ barrier is generally characterized by an increase in intestinal permeability, an important pathogenic factor contributing to the development of intestinal inflammation [20]. The activity of mucosal enzyme DAO is a sensitive marker of intestinal permeability, and serum DAO level is also found to serve as a useful indicator of the severity of mucosal injury [21]. Our study here showed the significantly elevated serum level of DAO following intestinal I/R insult, along with the severe intestinal mucosal damage, corroborating with the results of previous studies [722]. Pretreatment with curcumin markedly attenuated serum DAO level and improved morphological alterations to the intestinal mucosa, indicating that curcumin can ameliorate intestinal permeability or injury after I/R.
The TJ scaffolding protein ZO-1 has been well documented to play an important role in maintenance of intestinal mucosal barrier integrity, and disruption of the intestinal mucosa and the consequent increase in permeability after I/R injury may be due to reduced levels of ZO-1. Our immunohistochemical and western blot results showed that ZO-1 expression in the intestinal tissue significantly decreased after I/R injury. This finding is consistent with that of previous studies showing that intestinal I/R insult may result in the lower ZO-1 level that contributing to TJ disruption and possibly increase gut permeability [7]. On the other hand, pretreatment of I/R insult rats with curcumin significantly reversed I/R induced downregulation of ZO-1 protein. Intestinal reperfusion-induced tissue injury led to a perturbation in the antioxidant defense mechanism of the intestine [12]. The expression of ZO-1 was found to be downregulated in response to proinflammatory cytokines and redistributed away from the TJ during the increased permeability [823]. These inflammatory changes to the epithelial TJs have been shown to be partially mediated by TNF-α [724]. As an essential mediator of inflammation in the gut, TNF-α plays a central role in the physiological regulation of intestinal barrier function [2526]. Intestinal epithelium integrity is regulated by TNF-α concentration in the extracellular media [27]. Anti-TNF-α therapy can dramatically reduce gut inflammation and largely restore the intestinal barrier [28]. TNF-α is known to inhibit the expression of ZO-1 [29]. Evidence has been recently provided that TNF-α can induce an increase in TJ permeability and ZO-1 protein down-regulation [30], and bone-marrow mesenchymal stem cells may reduce rat intestinal I/R injury, ZO-1 down-regulation and TJ disruption via a TNF-α regulated mechanism [7]. The precise mechanisms involved in TNF-α regulation of ZO-1 expression have not been well-established. Ma et al. have indicated that TNF-α may cause an increased intestinal epithelial TJ permeability through activation of nuclear factor-κB (NF-κB), resulting in the downregulated expression of ZO-1 protein and altered junctional localization, and several possible scenarios have been proposed to clarify the TNF-α-regulated NF-κB activation and ZO-1 downregulation [30]. Research has indicated that curcumin can inhibit the generation of TNF-α in I/R intestinal tissue [12], and therefore we hypothesized that curcumin may repair intestinal I/R injury by inhibiting the release of TNF-α. In this study, serum TNF-α level was significantly higher in I/R group as determined by ELISA analysis. Our data were consistent with those of the previous studies, which indicated the markedly elevated serum TNF-α level during the increasing time of I/R injury [3132]. Curcumin addition in group III significantly reduced serum TNF-α level and increased the ZO-1 expression of intestinal tissues when compared with group II I/R subjected rats, along with the reduced histopathologic changes of I/R-induced intestinal mucosal injury. However, one thing worth noting was that unlike the results of our study and those of others, serum level of TNF-α was found not significantly changed after 45 min SMA ligation followed by 24 h reperfusion, and oral gavage of curcumin to I/R subjected rats for 20 days resulted in no significant change in serum TNF-α level [13]. The potential reason for these conflicting results is still unclear, and this effect can be partly explained by the animals used, the time and dose of curcumin administrated. Additionally, the way curcumin given and the details of surgical procedures may differently affect the results obtained.
In summary, our study showed evidence suggested that I/R insult by occlusion of the SMA resulted in a significant increase in intestinal permeability, the upregulated serum level of TNF-α, and the reduced expression of the TJ-associated protein, ZO-1. The present study showed evidence suggested that curcumin pretreatment may restore the epithelial structure, promote the recovery of intestinal permeability, increase ZO-1 protein expression, and protect against intestinal I/R injury partly through TNF-α related mechanism.
Notes
Author contributions: S.Y.T., R.X.G., and S.C.W. have contributed to the study conception and design. S.Y.T., R.X.G., Y.K., X.L.W., and W.W.W. have participated in the experimental performance. S.C.W., X.M.S., and H.Y.J. have helped to analyze and interpret the data, as well as write and revise the manuscript.
References
1. Berland T, Oldenburg WA. Acute mesenteric ischemia. Curr Gastroenterol Rep. 2008; 10:341–346. PMID: 18625147.

2. Douzinas EE, Pitaridis MT, Patsouris E, Kollias S, Boursinos V, Karmpaliotis DI, Gratsias Y, Evangelou E, Papalois A, Konstantinidou AE, Roussos C. Myocardial ischemia in intestinal postischemic shock: the effect of hypoxemic reperfusion. Crit Care Med. 2003; 31:2183–2189. PMID: 12973178.

3. Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001; 15:1–10.

4. Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000; 279:G250–G254. PMID: 10915631.

5. Li Q, Zhang Q, Wang C, Liu X, Qu L, Gu L, Li N, Li J. Altered distribution of tight junction proteins after intestinal ischaemia/reperfusion injury in rats. J Cell Mol Med. 2009; 13:4061–4076. PMID: 19929946.

6. González-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol. 2000; 11:315–324. PMID: 10966866.

7. Shen ZY, Zhang J, Song HL, Zheng WP. Bone-marrow mesenchymal stem cells reduce rat intestinal ischemia-reperfusion injury, ZO-1 downregulation and tight junction disruption via a TNF-α-regulated mechanism. World J Gastroenterol. 2013; 19:3583–3595. PMID: 23801859.
8. Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007; 140:12–19. PMID: 17418867.

9. Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010; 103:1545–1557. PMID: 20100380.
10. Cho YJ, Yi CO, Jeon BT, Jeong YY, Kang GM, Lee JE, Roh GS, Lee JD. Curcumin attenuates radiation-induced inflammation and fibrosis in rat lungs. Korean J Physiol Pharmacol. 2013; 17:267–274. PMID: 23946685.

11. Kim KC, Lee C. Curcumin induces downregulation of E2F4 expression and apoptotic cell death in HCT116 human colon cancer cells; involvement of reactive oxygen species. Korean J Physiol Pharmacol. 2010; 14:391–397. PMID: 21311680.

12. Karatepe O, Gulcicek OB, Ugurlucan M, Adas G, Battal M, Kemik A, Kamali G, Altug T, Karahan S. Curcumin nutrition for the prevention of mesenteric ischemia-reperfusion injury: an experimental rodent model. Transplant Proc. 2009; 41:3611–3616. PMID: 19917353.

13. Nurullahoglu-Atalik KE, Okudan N, Belviranli M, Gokbel H, Oz M, Esen H. Role of curcumin in mesenteric ischemia-reperfusion injury in rats. Bratisl Lek Listy. 2012; 113:465–470. PMID: 22897369.
14. Yucel AF, Kanter M, Pergel A, Erboga M, Guzel A. The role of curcumin on intestinal oxidative stress, cell proliferation and apoptosis after ischemia/reperfusion injury in rats. J Mol Histol. 2011; 42:579–587. PMID: 21984066.

15. Sahebkar A. Molecular mechanisms for curcumin benefits against ischemic injury. Fertil Steril. 2010; 94:e75–e76. PMID: 20797714.

16. Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970; 101:478–483. PMID: 5457245.
17. Jiang H, Qu L, Li Y, Gu L, Shi Y, Zhang J, Zhu W, Li J. Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats. J Surg Res. 2011; 168:127–134. PMID: 19932900.

18. Deitch EA, Morrison J, Berg R, Specian RD. Effect of hemorrhagic shock on bacterial translocation, intestinal morphology, and intestinal permeability in conventional and antibioticdecontaminated rats. Crit Care Med. 1990; 18:529–536. PMID: 2328600.

19. Costantini TW, Deree J, Loomis W, Putnam JG, Choi S, Baird A, Eliceiri BP, Bansal V, Coimbra R. Phosphodiesterase inhibition attenuates alterations to the tight junction proteins occludin and ZO-1 in immunostimulated Caco-2 intestinal monolayers. Life Sci. 2009; 84:18–22. PMID: 18992758.

20. Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009; 58:41–48. PMID: 18829978.

21. Hou Y, Wang L, Zhang W, Yang Z, Ding B, Zhu H, Liu Y, Qiu Y, Yin Y, Wu G. Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids. 2012; 43:1233–1242. PMID: 22180025.

22. Sun Q, Meng QT, Jiang Y, Liu HM, Lei SQ, Su WT, Duan WN, Wu Y, Xia ZY, Xia ZY. Protective effect of ginsenoside Rb1 against intestinal ischemia-reperfusion induced acute renal injury in mice. PLoS One. 2013; 8:e80859. PMID: 24324637.

23. Musch MW, Walsh-Reitz MM, Chang EB. Roles of ZO-1, occludin, and actin in oxidant-induced barrier disruption. Am J Physiol Gastrointest Liver Physiol. 2006; 290:G222–G231. PMID: 16239402.

24. Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005; 288:G422–G430. PMID: 15701621.
25. Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005; 166:409–419. PMID: 15681825.
26. Al-Sadi R, Guo S, Ye D, Ma TY. TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol. 2013; 183:1871–1884. PMID: 24121020.

27. Song HL, Lu S, Ma L, Li Y, Liu P. Effect of TNF-α on tight junctions between the epithelial cells of intestinal mucosal barrier. World Chin J Digestol. 2004; 12:1303–1306.

28. Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol. 2002; 97:2000–2004. PMID: 12190167.

29. Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005; 19:923–933. PMID: 15923402.
30. Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004; 286:G367–G376. PMID: 14766535.
31. Grootjans J, Lenaerts K, Derikx JP, Matthijsen RA, de Bruïne AP, van Bijnen AA, van Dam RM, Dejong CH, Buurman WA. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol. 2010; 176:2283–2291. PMID: 20348235.
32. Yang Q, Zheng FP, Zhan YS, Tao J, Tan SW, Liu HL, Wu B. Tumor necrosis factor-α mediates JNK activation response to intestinal ischemia-reperfusion injury. World J Gastroenterol. 2013; 19:4925–4934. PMID: 23946597.

Fig. 1
Effect of curcumin pretreatment on the intestinal I/R injury.
Histopathological findings of H&E staining shows normal histopathology of the sham group (A), Intestinal I/R insult results in the denuded villi and architectural disintegration (B), Curcumin pretreatment partly ameliorates this I/R-induced damage to the intestinal tissue (C). I/R, ischemia reperfusion; H&E, Haematoxylin & Eosin.
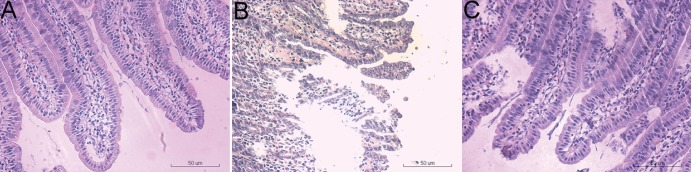
Fig. 2
Effect of curcumin pretreatment on intestinal expression of TNF-α protein after I/R as determined by Western blot assay.
I/R, ischemia reperfusion; TNF-α, Tumor Necrosis Factor-α.

Fig. 3
Effect of curcumin pretreatment on intestinal expression of ZO-1 protein after I/R as determined by immunohistochemical assay.
ZO-1 expressions in intestinal tissues of the sham operated group (A), I/R injury group (B), and curcumin-pretreated I/R injury one (C). I/R, ischemia reperfusion; ZO-1, Zonula occludens protein-1.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download