Abstract
Damage in the periphery or spinal cord induces maladaptive plastic changes along the somatosensory nervous system from the periphery to the cortex, often leading to chronic pain. Although the role of neural circuit remodeling and structural synaptic plasticity in the 'pain matrix' cortices in chronic pain has been thought as a secondary epiphenomenon to altered nociceptive signaling in the spinal cord, progress in whole brain imaging studies on human patients and animal models has suggested a possibility that plastic changes in cortical neural circuits may actively contribute to chronic pain symptoms. Furthermore, recent development in two-photon microscopy and fluorescence labeling techniques have enabled us to longitudinally trace the structural and functional changes in local circuits, single neurons and even individual synapses in the brain of living animals. These technical advances has started to reveal that cortical structural remodeling following tissue or nerve damage could rapidly occur within days, which are temporally correlated with functional plasticity of cortical circuits as well as the development and maintenance of chronic pain behavior, thereby modifying the previous concept that it takes much longer periods (e.g. months or years). In this review, we discuss the relation of neural circuit plasticity in the 'pain matrix' cortices, such as the anterior cingulate cortex, prefrontal cortex and primary somatosensory cortex, with chronic pain. We also introduce how to apply long-term in vivo two-photon imaging approaches for the study of pathophysiological mechanisms of chronic pain.
Chronic pain is a major challenge to clinical practice as well as basic neuroscience [1]. Tissue or nerve injury triggers or induces plastic changes in peripheral nociceptive nerve endings, in the dorsal horn of the spinal cord, as well as in supraspinal and cortical areas [23]. All of these changes in the nervous system may contribute to increased pain sensitivity [45]. Compared with functional plasticity alone, structural alteration could augment the memory storage capacity as structural rewiring allows more variability and therefore a larger number of potential circuits to be generated, implying a larger memory capacity per synapse [6]. Several studies have demonstrated that chronic pain is closely associated with structural changes in the central nervous system such as the spinal cord and brain [78]. Considering the similar mechanism of memory and chronic pain, exemplified by "long-term potentiation" and "central sensitization", neural circuit remodeling might have an important affect to the perception of pain [9].
Many research papers on the relation of chronic pain and neural structure in the central nervous system have been published. It is mostly focusing on the alteration of neurons in the spinal cord. The sprouting of A-fibers from deep to superficial lamina one week following traumatic nerve injury has been proposed to drive neuropathic pain hypersensitivity since several decades [1011]. However, this suggestion is now controversial because recent studies from different laboratories have demonstrated that such sprouting of low-threshold A-fiber mechanoreceptors does not occur [1213]. In fact, large-scale organization of axonal and dendritic arbors seems to be very stable, compared to the dynamic changes in synaptic connectivity through the growth and elimination of synaptic structures (i.e., axonal bouton and dendritic spine) [14]. Waxman and his colleagues have conducted a series of experiment on remodeling of dendritic spines in the spinal cord of different pain and injury models. Ten days after peripheral nerve injury, spine density increased with mature, mushroom-shaped spines preferentially distributed along dendritic branch regions closer to the cell body [15]. In a diabetic neuropathic pain model, they have reported alteration in dendritic spine shape and distribution on wide-dynamic-range neurons within lamina IV~V of the dorsal horn [16]. In a spinal cord injury (SCI) induced chronic pain rat model, the conversion from thin-shaped to more mature, mushroom-shaped spine structures results in enhanced synaptic transmission and fidelity, improved frequency-following ability, and reduced inhibitory gating effectiveness [17].
In the brain, preliminary evidence of pain-related structural changes was initially revealed by functional magnetic resonance imaging (fMRI) mostly concentrated on human adult brain. Imaging studies showing altered brain morphology and connectivity in multiple types of chronic pain conditions, such as chronic back pain [18] fibromyalgia [19] complex regional pain syndrome [20] and headache [21], have been reported. However, as most of these studies were conducted based on images with limited spatial resolution and temporal resolution, changes in local neural circuits of individual neurons and synapses in the brain are not clearly demonstrated. Recently, long-term in vivo two-photon imaging represents a newly developed tool for studying the structural changes in the brain of living animals [2223] and longitudinal observation of individual neurons and synapses is enabled. Compare to the large number of human imaging studies published, only a handful of previous studies have been done to assess the structural correlates of chronic pain in the brain of animals.
Hitherto, a certain amount of investigation has been conducted, and this review is written to report the results that have been produced and help further research. Here, based on articles focused on the relation of chronic pain to structural plasticity, we will discuss changes in different parts of the brain, such as the amygdala, anterior cingulate cortex (ACC), hippocampus, prefrontal cortex (PFC), primary somatosensory cortex (S1) and thalamus. Furthermore, recent advance in two-photon imaging studies will be introduced as rewiring of local neural circuits can be observed.
Recent experiments conducted in both human patients and animal models have demonstrated that the presence of chronic pain is closely associated with significant functional and structural modifications in different cortical regions, including the amygdala, ACC, hippocampus, PFC and S1.
In an article of Apkarian et al., six most common regions activated in acute pain are often referred to as the pain matrix. These regions includes: S1, secondary somatosensory cortex (S2), ACC, insular cortex (IC), PFC and the thalamus [24]. However in chronic pain, Lithwick et al. state that only five regions are included in the pain matrix with the exception of thalamus, which is not associated with the processing of pain, but rather relays sensory information to cortical and subcortical structures [25]. Although it was not in a chronic pain model, Takeuchi et al. have observed rapid rewiring of afferent fibers in the mature ventral posteromedial thalamic nucleus of mice after transection of the peripheral whisker sensory nerve, using the whole-cell voltage-clamp technique [26]. Also, Draganski et al. have reported a loss of thalamic gray matter in 28 volunteers with unilateral limb amputation using voxel-based morphometry, which is based on high-resolution magnetic resonance images [27]. They suggested that the loss of neurons might be a secondary response to injury, rather than a cause of chronic pain, reporting that the decrease of gray matter is related to the duration of amputation, but not to the amount of phantom pain.
In this review, S2 and IC have not been mentioned as, to our best knowledge, no articles have been found on the neuronal structure plasticity concerning this area. However, several articles have reported that S1 and S2 is closely related and we supppose that the structural changes in the S2 might occur along with S1 area, although in one study, after having examined cortical reorganization in response to phantom pain treatment, S1 exhibited cortical reorganization while S2 did not [28]. Also, the IC is considered to be related to both sensory and affective perception of pain, and some articles have reported its relation with the ACC [29]. Reorganized connectivity between the PFC and the IC have been observed in a chronic back pain patient with fMRI [30]. In addition to S2 and IC, neuronal plasticity in the motor cortex has been reported in an article by Kim et al. [31]. Using confocal microscopy in fixed slices, they have observed dendritic spines in the rat motor cortex after an overhemisection injury at C4 level. Spine density decreased at 7 days and partially recovered by 28 days and spine head diameter significantly increased in a layer-specific manner.
The amygdala is an almond-shaped structure in the medial temporal lobe in the limbic system that plays an important role in behavioral responses to emotional stimuli [32]. It is also known to be deeply involved in the processing of the emotional component of pain, probably through a modulatory role upon major supraspinal pain control centers [33]. It does not only modulate pain through the descending inhibitory control systems but also contributes to the generation and enhancement of pain responses [34].
In an article by Tajerian et al., mice with chronic pain induced by fracture/cast show increased complexity in dendritic structure in the contralateral amygdala compared to control group mice. The difference was shown by the mean number of intersections of dendritic branches with consecutive 5-µm-spaced concentric sholl rings. Furthermore, independent quantification of neuronal structure using direct counts revealed that the fracture/cast group has a significantly greater number of secondary nodes compared with control, indicative of increased dendritic branching. No differences were seen in measures of dendritic length, average soma area, total number of neurons, or dendritic spine density [35].
Goncalves et al. showed an increased amygdala volume in spared nerve injury (SNI) model rats. Additional morphological analysis suggested that increased cell number, but not increased cell volume and dendritic length, contributes to the enlarged amygdala volume in the SNI treated rats. They further suggest that these neural circuit modification of the amygdala might correlate with the development of depressive-like behavior in animals with neuropathic pain [36].
The ACC is a major cortical area that is believed to contribute to a myriad of brain functions, including attention, learning, memory, emotion and pain processing [37]. Zhuo M. states that ACC along with IC is an important cortical region responding to physiological and pathological pain, which is critical for pain perception, and that evidence indicates that plasticity in the ACC is involved in the development of persistent pain [38]. Zhuo and his colleagues have shown that long-term presynaptic and postsynaptic change occurs in cortical synapses after nerve injury and that these changes were absent in genetic mice lacking calcium-stimulated adenylyl cyclase 1 [39]. Eto et al. have also demonstrated using in vivo two-photon Ca2+ imaging that intra-regional remodeling within S1 of the mouse accelerates chronic pain behavior by modulating neuronal activity in the ACC [22].
Blom et al. performed multiple whole-cell recordings from neurons in layer V of the ACC of adult mice after chronic constriction injury of the sciatic nerve of the left hind paw and observed a striking loss of connections between excitatory and inhibitory neurons in both directions [40]. They suggested that cortical network is disinhibited and this might explain the increased activity observed in the ACC in patients with nerve injury. Also, in a human placebo study, a decrease in ACC activation along with the anterior insula correlated with a decrease in pain [41].
The hippocampus, a central component of the limbic system involved in regulation of spatial memory and mood or affect, is one of several brain regions that is capable of continuous cell proliferation and neurogenesis throughout adulthood in humans and other animals [42]. Several studies have reported the relation of hippocampal structure plasticity to chronic pain. Human patient case study published by Zimmerman et al. [43] states that the hippocampal volume is reduced in elderly individuals with chronic pain. In animal models of chronic pain, Duric and McCarson examined the hippocampal morphology with bromodeoxyuridine (BrDU) staining and found that neurogenesis in the dentate gyrus (DG) area was significantly reduced after CFA-induced long-term inflammatory nociception but not after formalin elicited acute nociception [42]. Tareda et al. evaluated the environmental enrichment (EE)-induced hippocampal neurogenesis in nerve-ligated mice. Mice were housed either in a standard environment or in the EE for 4 weeks. In sham control group, EE increased the immunoreactivity for doublecortin, a marker for immature neuron-positive cells, as well as NeuroD (a neurogenic basic helix-loop-helix factor)-positive cells in the DG, compared to standard environment. However, nerve-ligation induced injury suppressed EE mediated induction of both DCXand NeuroD-labeled cells [44].
The reduction of presynaptic boutons in hippocampal CA1 region was observed by Ren et al. By calculating the density of synaptophysin-positive puncta in the CA1 area, they demonstrated that SNI in both rats and mice reduced the presynaptic terminal puncta density, a form of ultrastructural plasticity that was supposed to be correlated with altered short-term plasticity (frequency facilitation) at CA3-CA1 synapses [45]. In addition, Mutso et al. found a robust decrease in the hippocampal neurogenesis 14 days after SNI as evidenced by reduced number of BrdU positive cells in bilateral DG [46].
The PFC is associated with high-order cognitive and emotional functions including attention, decision making, goal-directed behavior, and working memory [47]. Among different subregions having a role in the processing of pain, the medial PFC was found to be involved in signaling the unpleasantness of pain.
Using the SNI neuropathic pain model rats, Metz et al. performed patch-clamp recordings and anatomical analysis of layer II/III pyramidal neurons in the contralateral medial PFC. They reported that basal dendrites of the pyramidal cells in the medial PFC were much longer and had more branches than their counterparts from the sham control group. Spine density was also selectively increased in basal dendrites of neurons from SNI rats, without any significant change for the apical dendrites. Metz et al. further state that the changes occurred only in basal dendrites may suggest the reorganization of intracortical circuits rather than the reorganization of extracortical inputs [48].
Earlier findings from macroscopic brain imaging studies have suggested that maladaptive plastic changes, such as hyperexcitability and reorganization, in the S1 cortex play active roles in the chronification of neuropathic pain [4950].
Florence et al. have shown the evidence of axonal reorganization in the S1 cortex in amputated adult macaque monkeys. Using the neuronal labeling and microelectrode recording, they have proved that massive change, such as axonal sprouting, occurs in the cortex related to the affected limb [51]. Furthermore, with the use of microelectrode mapping techniques Merzenich et al. have demonstrated the functional and structural change of S1 2~8 months after surgical amputation of digit in adult monkeys [52]. Such large-scale organization of axonal and dendritic arbors, however, is rarely seen in longitudinal imaging of the intact adult cortex over weeks [5354], even following peripheral nerve injury (Fig. 1b). Instead, in vivo two-photon imaging studies have started to reveal that neural circuit remodeling that is responsible for storing specific long-term information following novel sensory experiences or peripheral injury could be achieved by subtle changes in the formation and elimination of synaptic structures [145556].
To observe the plastic changes in synapse morphology, formation and elimination in the S1 area in animal models of pain, Nabekura, Kim and his colleagues used long-term in vivo two-photon imaging [5657]. First, they reported that partial sciatic nerve ligation resulted in a phase-specific and size-dependent growth/survival of newly formed dendritic spines and previously persistent spines, revealing a rapid and selective remodeling of cortical synapses that provides the structural correlate of neuropathic pain in the S1 ([56], see also Fig. 1c). Subsequently, using the short-term in vivo two photon imaging, they further demonstrated that spine motility and the proportion of immature (thin) spines significantly increased in the development (but not maintenance) phase of neuropathic pain. Also, the formation and elimination of axonal boutons moderately increased and decreased, respectively, during the first 3 days following the nerve injury [57]. In an experiment using in vivo two-photon Ca2+ imaging conducted by Eto et al., they have reported that the spontaneous activity of layer II/III neurons in the S1 and the evoked response to sensory and layer IV stimulations increased under chronic inflammatory pain conditions [22].
Putting all these results together, it is suggested that peripheral nerve injury can indeed elicit rapid and dynamic neural circuit rewiring in the S1 area, which may provide a novel target for treatment of intractable neuropathic pain.
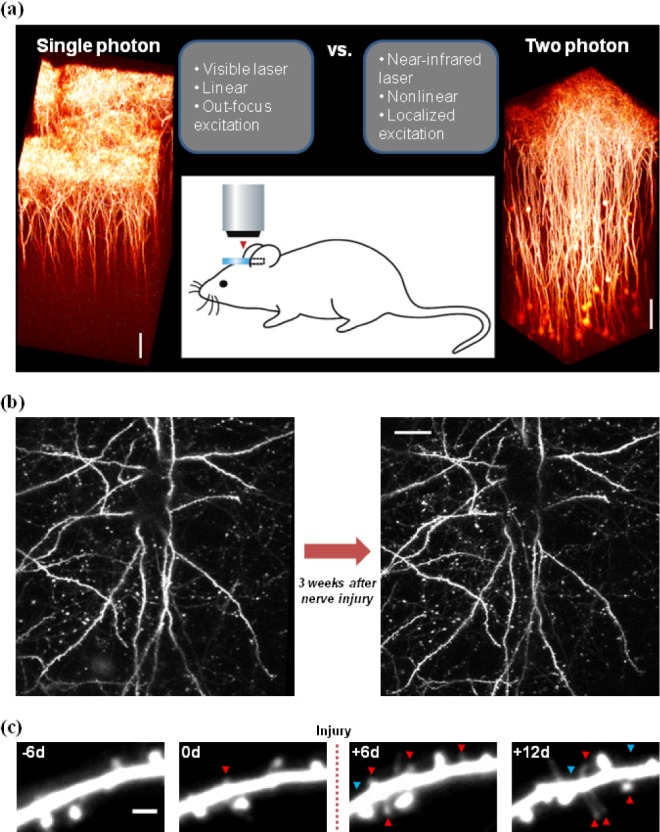
Technical advances in two-photon laser scanning microscopy and fluorescence labeling methods have enabled researchers to directly observe the structural and functional plastic changes in neuronal populations at single cell or individual synapse level [142358]. The advantages of two-photon microscopy imaging are shown in Fig. 1a, compared to conventional single photon microscopy: (i) use of near-infrared laser (vs. visible laser) enabling deep tissue imaging (~1 mm) by low scattering; (ii) nonlinear excitation properties of fluorescent molecules (vs. linear), i.e. increasing the laser intensity twice results in 4 times increase of emission signals, enabling relatively low laser intensity to be sufficient for imaging; (iii) localized excitation (vs. out-focus excitation) enabling high resolution imaging with no requirement of confocal pinhole that causes some loss of emission signals. The latter two advantages that also contribute to less photodamage, together with the former advantage for deep tissue imaging, make the two-photon microscopy optimal for long-term in vivo imaging of neurons and synapses in the intact brain (for detailed explanations about the principle of two-photon microscopy in a biology field, see review articles [585960]).
Recently, research on neural circuit remodeling under chronic pain is seen as a major clue to understand the mechanism of pain and evidence has been accumulating to demonstrate that multiple types of chronic pain-related comorbidities implicate both functional and structural synaptic plasticity. With the understanding of chronic pain, it has been more obvious that it is strongly associated with both emotional and sensory components of the brain. In this review, we have discussed the change of neurons and synapses in the cerebral cortex such as amygdala, ACC, hippocampus, PFC and S1 (summarized in Table 1). All these results included in this review demonstrate that chronic pain, regardless of the etiology (inflammatory or neuropathic) and pain model, is likely to trigger various forms of structural plasticity in the cortex, which in turn could be directly or indirectly involved in the development of sensory and emotional/cognitive symptoms of chronic pain.
It has long been believed that structural plasticity of neuronal connections in the brain occurs certain period (i.e., month or years) after the functional change (i.e., days or weeks) in chronic pain. However, as mentioned in articles included in this review, using long-term in vivo two-photon imaging, it is demonstrated that rapid structural reorganization of synaptic connections in the related sensory or motor cortex can take place within days or weeks correlated with functional plasticity. This may raise the importance of timing of intervention in preventing chronic pain [23].
In our review, plastic changes in the human brain are not mentioned in the table (Table 1) because most of these studies were conducted based on images with limited spatial and temporal resolution and changes in local neural circuits of individual neurons and synapses in the brain are not clearly demonstrated. We have tried to see the change at the early and late phase of chronic pain in each part of the brain mentioned in the text, but unfortunately, the relatively small number of published studies prevents closer examination of short and long term change in each part of the brain. Also, we are more focusing on the structural than functional plasticity of the cortex during chronic pain. Whether the included articles focus on the early or late phase of chronic pain and on structural or functional change is mentioned in the table (Table 1).
Still, whether neural circuit remodeling is the cause or the result of the chronic pain is not clearly understood. Although Rodriguex -raecke et al. state that the change in the brain is the consequence not the cause of the pain, this question still remains controversial [61]. In addition, many aspects of the maladaptive plastic changes in cortical neural circuits and synaptic structures remain unsolved. For example, the role of glial cells (e.g. microglia and astrocytes) in the cortical plasticity as well as in chronic pain symptomps are not fully understood compare to its action in the spinal cord. How and to what extend do cortical glial cells participate in the alteration during chronic pain needs furthur investigation. Also, how do several cortical 'pain matrix' regions influence each other and interact with spinal cord nociceptive circuits? Research in this field is still at its beginning, entailing more instructive future studies to gain our understanding of the structural substrate of chronic pain and the accompanying comorbidities.
ACKNOWLEDGEMENTS
We thank Dr. Junichi Nabekura for his kind support and advice on this manuscript. This work was supported by National Research Foundation of Korea grants funded by the Korea government (NRF-2013R1A1A1012403). Funders had no involvement in the study design, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
References
2. Saab CY. Pain-related changes in the brain: diagnostic and therapeutic potentials. Trends Neurosci. 2012; 35:629–637. PMID: 22763295.

3. Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, Curatolo M. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004; 107:7–15. PMID: 14715383.

4. Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000; 288:1765–1769. PMID: 10846153.

5. Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009; 87:81–97. PMID: 18952143.

6. Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004; 431:782–788. PMID: 15483599.

7. Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage. 2009; 47:1007–1014. PMID: 19497372.

9. Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003; 26:696–705. PMID: 14624855.

10. Proudlock F, Spike RC, Todd AJ. Immunocytochemical study of somatostatin, neurotensin, GABA, and glycine in rat spinal dorsal horn. J Comp Neurol. 1993; 327:289–297. PMID: 7678841.

11. Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992; 355:75–78. PMID: 1370574.

12. West SJ, Bannister K, Dickenson AH, Bennett DL. Circuitry and plasticity of the dorsal horn--toward a better understanding of neuropathic pain. Neuroscience. 2015; 300:254–275. PMID: 25987204.
13. Zhang Y, Chen Y, Liedtke W, Wang F. Lack of evidence for ectopic sprouting of genetically labeled Aβ touch afferents in inflammatory and neuropathic trigeminal pain. Mol Pain. 2015; 11:18. PMID: 25880319.

14. Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009; 10:647–658. PMID: 19693029.

15. Tan AM, Chang YW, Zhao P, Hains BC, Waxman SG. Rac1-regulated dendritic spine remodeling contributes to neuropathic pain after peripheral nerve injury. Exp Neurol. 2011; 232:222–233. PMID: 21963650.

16. Tan AM, Samad OA, Fischer TZ, Zhao P, Persson AK, Waxman SG. Maladaptive dendritic spine remodeling contributes to diabetic neuropathic pain. J Neurosci. 2012; 32:6795–6807. PMID: 22593049.

17. Tan AM, Choi JS, Waxman SG, Hains BC. Dendritic spine remodeling after spinal cord injury alters neuronal signal processing. J Neurophysiol. 2009; 102:2396–2409. PMID: 19692517.

18. Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004; 24:10410–10415. PMID: 15548656.

19. Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007; 27:4004–4007. PMID: 17428976.

20. Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008; 60:570–581. PMID: 19038215.

21. Schmidt-Wilcke T, Leinisch E, Straube A, Kämpfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005; 65:1483–1486. PMID: 16275843.

22. Eto K, Wake H, Watanabe M, Ishibashi H, Noda M, Yanagawa Y, Nabekura J. Inter-regional contribution of enhanced activity of the primary somatosensory cortex to the anterior cingulate cortex accelerates chronic pain behavior. J Neurosci. 2011; 31:7631–7636. PMID: 21613476.

23. Kim SK, Eto K, Nabekura J. Synaptic structure and function in the mouse somatosensory cortex during chronic pain: in vivo two-photon imaging. Neural Plast. 2012; 2012:640259. PMID: 22530157.
24. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005; 9:463–484. PMID: 15979027.

25. Lithwick A, Lev S, Binshtok AM. Chronic pain-related remodeling of cerebral cortex - 'pain memory': a possible target for treatment of chronic pain. Pain Manag. 2013; 3:35–45. PMID: 24645930.

26. Takeuchi Y, Yamasaki M, Nagumo Y, Imoto K, Watanabe M, Miyata M. Rewiring of afferent fibers in the somatosensory thalamus of mice caused by peripheral sensory nerve transection. J Neurosci. 2012; 32:6917–6930. PMID: 22593060.

27. Draganski B, Moser T, Lummel N, Gãnssbauer S, Bogdahn U, Haas F, May A. Decrease of thalamic gray matter following limb amputation. Neuroimage. 2006; 31:951–957. PMID: 16520065.

28. MacIver K, Lloyd DM, Kelly S, Roberts N, Nurmikko T. Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain. 2008; 131:2181–2191. PMID: 18567624.

29. Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009; 10:59–70. PMID: 19096369.
30. Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci. 2011; 31:13981–13990. PMID: 21957259.

31. Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol. 2006; 198:401–415. PMID: 16443221.

32. Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001; 6:13–34. PMID: 11244481.

33. Manning BH, Merin NM, Meng ID, Amaral DG. Reduction in opioid- and cannabinoid-induced antinociception in rhesus monkeys after bilateral lesions of the amygdaloid complex. J Neurosci. 2001; 21:8238–8246. PMID: 11588195.

34. Rhudy JL, Meagher MW. Negative affect: effects on an evaluative measure of human pain. Pain. 2003; 104:617–626. PMID: 12927634.

35. Tajerian M, Leu D, Zou Y, Sahbaie P, Li W, Khan H, Hsu V, Kingery W, Huang TT, Becerra L, Clark JD. Brain neuroplastic changes accompany anxiety and memory deficits in a model of complex regional pain syndrome. Anesthesiology. 2014; 121:852–865. PMID: 25093591.

36. Gonçalves L, Silva R, Pinto-Ribeiro F, Pêgo JM, Bessa JM, Pertovaara A, Sousa N, Almeida A. Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp Neurol. 2008; 213:48–56. PMID: 18599044.

37. Liu MG, Chen J. Preclinical research on pain comorbidity with affective disorders and cognitive deficits: Challenges and perspectives. Prog Neurobiol. 2014; 116:13–32. PMID: 24444673.

38. Zhuo M. Cortical excitation and chronic pain. Trends Neurosci. 2008; 31:199–207. PMID: 18329111.

39. Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci. 2008; 28:7445–7453. PMID: 18632948.

40. Blom SM, Pfister JP, Santello M, Senn W, Nevian T. Nerve injury-induced neuropathic pain causes disinhibition of the anterior cingulate cortex. J Neurosci. 2014; 34:5754–5764. PMID: 24760836.

41. Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004; 303:1162–1167. PMID: 14976306.

42. Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006; 7:544–555. PMID: 16885011.

43. Zimmerman ME, Pan JW, Hetherington HP, Lipton ML, Baigi K, Lipton RB. Hippocampal correlates of pain in healthy elderly adults: a pilot study. Neurology. 2009; 73:1567–1570. PMID: 19901248.

44. Terada M, Kuzumaki N, Hareyama N, Imai S, Niikura K, Narita M, Yamazaki M, Suzuki T, Narita M. Suppression of enriched environment-induced neurogenesis in a rodent model of neuropathic pain. Neurosci Lett. 2008; 440:314–318. PMID: 18565655.

45. Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology. 2011; 36:979–992. PMID: 21289602.

46. Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012; 32:5747–5756. PMID: 22539837.

47. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001; 98:4259–4264. PMID: 11259662.

48. Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009; 106:2423–2428. PMID: 19171885.

49. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009; 32:1–32. PMID: 19400724.
50. Seifert F, Maihöfner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci. 2009; 66:375–390. PMID: 18791842.

51. Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998; 282:1117–1121. PMID: 9804549.

52. Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984; 224:591–605. PMID: 6725633.

53. Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002; 420:788–794. PMID: 12490942.

54. De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006; 49:861–875. PMID: 16543134.

55. Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009; 71:261–282. PMID: 19575680.

56. Kim SK, Nabekura J. Rapid synaptic remodeling in the adult somatosensory cortex following peripheral nerve injury and its association with neuropathic pain. J Neurosci. 2011; 31:5477–5482. PMID: 21471384.

57. Kim SK, Kato G, Ishikawa T, Nabekura J. Phase-specific plasticity of synaptic structures in the somatosensory cortex of living mice during neuropathic pain. Mol Pain. 2011; 7:87. PMID: 22067412.

58. Nemoto T. Living cell functions and morphology revealed by two-photon microscopy in intact neural and secretory organs. Mol Cells. 2008; 26:113–120. PMID: 18594180.
59. Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003; 21:1369–1377. PMID: 14595365.

60. Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005; 2:932–940. PMID: 16299478.

61. Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000; 28:41–51. PMID: 11086982.

Fig. 1
In vivo two-photon microscopy imaging in the cortex of living animals.
(a) Comparison of conventional single photon microscopy and two-photon microscopy imaging in the intact cortex of YFP-H line mouse [61], expressing yellow fluorescent proteins (YFPs) in many layer V pyramidal neurons. [Left] Representative 3D reconstructed single photon (confocal) microscopy image of layer V pyramidal neurons in the S1 cortex of anesthetized YFP-H mouse. Note that only distal dendrites, not proximal dendrites and cell bodies, of layer V pyramidal neurons can be seen up to 200~300 µm deep from the surface, for which full laser power should be used. Scale bar, 100 µm. [Middle-top] Characteristics of single photon imaging (left) and two-photon imaging (right). [Middle-bottom] Schematic drawing of in vivo two-photon imaging of neurons in a living mouse. [Right] Representative 3D reconstructed two-photon microscopy image of layer V pyramidal neurons in the S1 cortex of anesthetized YFP-H mouse. Note that whole parts of dendrites and cell bodies of layer 5 pyramidal neurons are clearly seen up to 600 µm deep from the surface. Scale bar, 100 µm. (b) Little change in dendritic arbors in nerve injured mice over 3 weeks. [Left] Z-projection image of apical dendrites (512×512 pixels, 0.62 µm/pixels, 50 optical planes, 1 µm step, 10~60 µm below the surface) in the S1 cortex taken just before peripheral nerve injury. [Right] Image of the same dendrites taken after peripheral nerve injury. Scale bar, 50 µm. (c) Representative images of the same dendrite in the S1 cortex taken before and after peripheral nerve injury. Arrowheads indicate dendritic spines generated (red) or eliminated (blue) when compared with the previous imaging session. Note the increase of spine formation and elimination after peripheral nerve injury. Scale bar, 2 µm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download