INTRODUCTION
METHODS
Animals
Murine asthma model
Solutions and Chemicals
Preparation of lung slices
Image acquisition and analysis
Statistical analysis
RESULTS
Lung inflammation and AHR in the murine asthma model
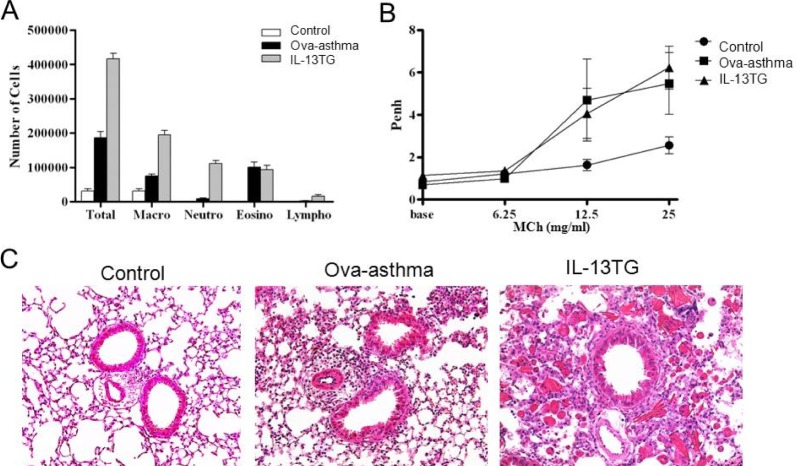 | Fig. 1Airway inflammation and hyperresponsiveness in murine asthma model. Increased total and differential cell count in bronchoalveolar lavage (BAL) fluid (A) and enhanced airway hyperresponsiveness (Penh) [15] (B) in Ova sensitized and challenged murine Ova-asthma and IL-13TG compared with untreated control. Lung histology shows increased peribronchial and perivascular infiltration of inflammatory cells in Ova-asthma and IL-13TG (C). |
Airway constrictions in PCLS
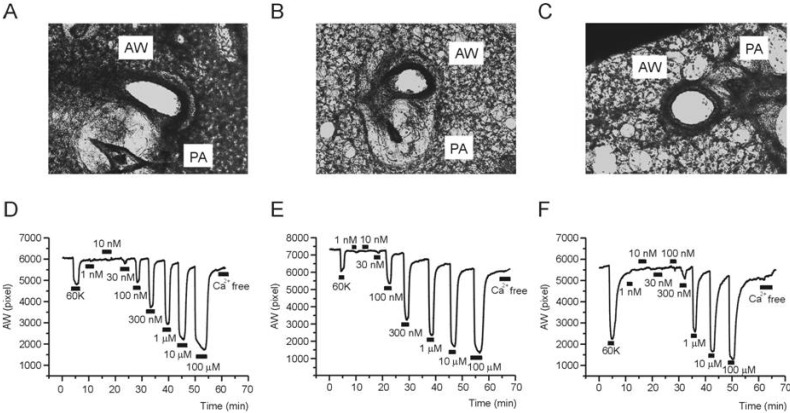 | Fig. 2Representative cases of PCLS experiment. Images of mouse lung slices taken from PCLS preparations; Control (A), Ova-asthma (B), and IL-13TG (C). Representative recordings of airway area (pixel number, AW) changes in response to 60K-induced depolarization and different concentrations of MCh in Control (D), Ova-asthma (E), IL-13 TG (F). |
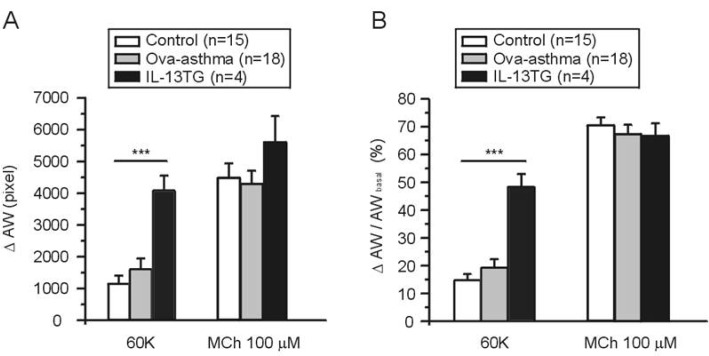 | Fig. 3AW change (ΔAW) during 60K-contraction and 100 µM MCh application. Summary of ΔAW in Control (n=15), Ova-asthma (n=18) and IL-13TG (n=4) are displayed as bar graphs (mean±SEM). Numbers of tested slices are also directly indicated above the figure. Ova-asthma had a tendency of higher sensitivity to 60 K (***p<0.001) in both ΔAW area (pixel number) changes (A) and ΔAW normalized to each basal (B, ΔAW/AWbasal). However, there were no differences in the maximum stimulation of muscarinic receptor among three groups. |
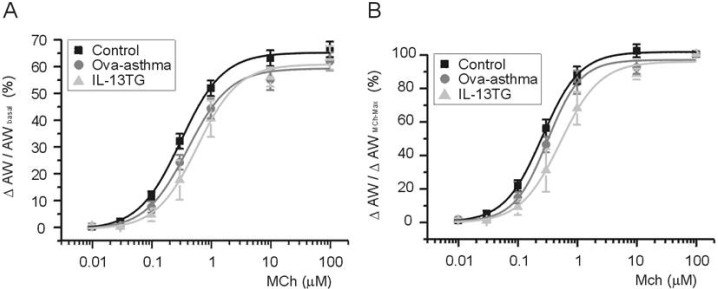 | Fig. 4Pharmacological sensitivity of AW responses to muscarinic stimulations. Concentration-response curves to differential concentrations of MCh. For comparison between groups, ΔAW normalized to each basal (A, ΔAW/AWbasal) and to maximum ΔAW-induced by 100 µM MCh (B, ΔAW/AWMChMax). Number of tested slices for the each group is same with that of figure 3. |
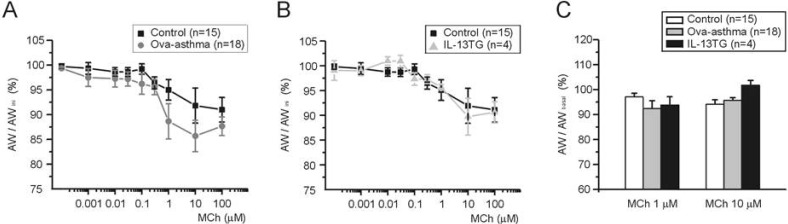 | Fig. 5Recovery AW after repetitive and incremental application of MCh. AWs were measured at 3 min after washout of various concentrations of MCh (see Fig. 2D-F). Then the measured AW was normalized to the initial AW in each slice (AW/AWini, A and B) or to the AW measured just before the application of MCh (C, AW/AWbasal). Comparison of control vs. Ova-asthma (A), and of control vs. IL-13TG (B). In C, summary of AW/AWbasal (%) for 1 and 10 µM of MCh in Ova-asthma and IL-13TG are shown as bar graphs. Albeit the apparent difference between control and Ova-asthma at 1 µM of MCh (A), statistical comparison shows p>0.05. Numbers of tested slices are directly indicated in figure. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download