Abstract
Retinyl palmitate (RP)-loaded pectinate micro- and nano-particles (PMP and PNP) were designed for stabilization of RP that is widely used as an anti-wrinkle agent in anti-aging cosmeceuticals. PMP/PNP were prepared with an ionotropic gelation method, and anti-oxidative activity of the particles was measured with a DPPH assay. The stability of RP in the particles along with pectin gel and ethanolic solution was then evaluated. In vitro release and skin permeation studies were performed using Franz diffusion cells. Distribution of RP in each skin tissue (stratum corneum, epidermis, and dermis) was also determined. PMP and PNP could be prepared with mean particle size diameters of 593~843 μm (PMP) and 530 nm (i.e., 0.53 μm, PNP). Anti-oxidative activity of PNP was greater than PMP due largely to larger surface area available for PNP. The stability of RP in PMP and PNP was similar but much greater than RP in pectin bulk gels and ethanolic solution. PMP and PNP showed the abilities to constantly release RP and it could be permeated across the model artificial membrane and rat whole skin. RP was serially deposited throughout the skin layers. This study implies RP loaded PMP and PNP are expected to be advantageous for improved anti-wrinkle effects.
Go to : 
Anti-wrinkle agents have worldwide been receiving significant interest as active materials to be incorporated into anti-aging cosmeceutical products and dermatological preparations [1]. One of the most widely used anti-wrinkle agents is known to be retinyl palmitate (RP), an ester form of retinol (vitamin A) and palmitic acid [2]. RP can stimulate the fibroblast to produce collagen, elastin, and hyaluronic acid, thereby reducing and preventing wrinkles when absorbed to the skin where it is enzymatically hydrolyzed to retinol, and further metabolized to retinoic acid that ultimately has the anti-wrinkle activity [3,4].
However, RP is generally considered to be chemically unstable, and among the chemical instabilities oxidation mainly caused by light irradiation is the most problematic issue in the stability of RP although it is more stable than its parent compound, retinol. One study has indeed reported a 38% loss of RP during the storage period of 7 days at even 4℃ [5]. Accordingly, the instability of RP in cosmeceutical products during storage is expected to lead to a decrease in its activity in the skin. Thus, an increase in the stability of RP would certainly be advantageous to obtain more pronounced anti-wrinkle performance.
Earlier approaches for stabilizing RP have usually focused on protection from photo oxidization by blocking UV light [6,7]. As reported in Carlotti's work [7], liposomes are one of the promising tools to stabilize RP but it is considered that they cannot securely enclose RP and moreover, liposomes are also easily oxidized, probably facilitating the oxidation of RP. We therefore screened anti-oxidative action of some polysaccharides in our previous study as efforts to identify a more effective stabilizer suitable for cosmeceutical products of RP. Of the polysaccharides tested, pectin demonstrated considerably stronger stabilizing ability [8]. The reason for the consideration of polysaccharides as stabilizers for RP was their usefulness in being formulated into cosmetic formulations such as gels, creams, and packs. In addition, we also confirmed the stabilizing action of pectin can improve the skin penetration and distribution characteristics of RP in a rat skin model [9]. Based on our previous investigation, we could further anticipate that microor nano-particulated RP can maximize the stabilizing effect of pectin on RP.
Herein, we explored pectinate micro-/nano-particulate systems could be used as the formulation because RP entrapped in the particulate system can be efficiently protected from the oxidative environment along with the stabilizing activity of pectin. Thus, in the present study, we prepared and characterized RP-loaded pectinate micro-/nano- particles (PMP/PNP), and performed RP stability, in vitro permeation, and skin transport studies test using RP-loaded PMP/PNP.
Go to : 
Pectin from apple with 70~75% degree of esterification, RP and α,α-diphenyl-β-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich Company (St. Louis, USA). Male Wistar rats (300~330 g, 8 weeks) were purchased from Hanlim Experimental Animals (Hwasung, Korea). All other chemicals were of analytical or high performance liquid chromatography (HPLC) grade.
To prepare RP-loaded PMP/PNP, 0.1 mL of RP ethanolic solution (10 mg/mL) was mixed with 0.9 mL of pectin aqueous solution (30 mg/mL). RP-loaded PMP were then prepared using an ionotropic gelation technique, with some modification [10]. The RP and pectin solution filled in a syringe was added dropwise to aqueous calcium chloride solution (5 mg/mL) using a syringe pump and a stream of nitrogen gas. The PMP formed were maintained in the calcium chloride solution for 10 min. RP-loaded PNP were prepared by introducing the RP and pectin solution mixture to a high pressure homogenizer (Nano Debee, BEE International Inc., MA, USA) along with a flow of aqueous calcium chloride solution (5 mg/mL). The homogenization was performed 3 cycles at 5000 psi. The PNP produced was then separated by centrifugation (8000 g, 10 min, 20℃).
Approximately, 30 RP-loaded PMP prepared above were randomly chosen and placed on a glass slide, then observed using a light microscope (S38, Microscopes INC., St. Louis, MO, USA). Microscopic observation of PNP could not be performed due to lack of suitable equipment. Instead of this particle size analysis demonstrated the formation of nanoparticles containing RP.
Particle distribution of RP-loaded PMP was determined with a sieving technique. The PMP were placed on the uppermost sieve of 5 sieves with aperture sizes of 300, 500, 710, 850, and 1500 µm, respectively, serially stacked. The 5 sieves were gently shaken to sort the PMP into each size range, followed by weighing the particles with each size range. To measure particle size distribution of RP-loaded PNP, PNP suspension appropriately diluted was analyzed with photon correlation spectroscopy using Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK).
RP-loaded PMP or PNP (approximately 30 mg) were dissolved in phosphate buffer (5 mL, pH 7.4) containing 5 mM EDTA. RP was then solubilized with ethanol (100 mL), and the amount of RP in samples was measured by HPLC. The entrapment efficiency and loading efficiency of RP in the PMP/PNP were then determined using below formulas:
where, We is the amount of RP entrapped in the PMP or PNP and Wi is the quantity of RP initially added during preparation procedures. Wp is the amount of pectin added.
Anti-oxidative effect of PMP and PNP was evaluated with a DPPH (1,1-diphenyl-2-picrylhydrazyl) assay [11]. One gram of PMP 1, PMP 2 (Table 1) and PNP was suspended in a DPPH solution (3 mL), respectively. The suspensions were well shaken and incubated for 30 min at room temperature. The incubated suspensions were then filtered through a nylon membrane with 0.45 µm pore size. The absorbance of the filtrates at 517 nm was measured using a microplate absorbance spectrophotometer. The DPPH radical scavenging effect was calculated by an equation below.
The stability test of RP in PMP 2, PNP suspension, pectin gel (0.5 mg pectin/mL) and RP ethanolic solution was performed. The 4 different formulations placed in glass vials with rubber stoppers were incubated at 40℃ for 8 h and samples were taken every 2 h from the vials. For RP-loaded PMP 2 and PNP, the samples were dissolved in 5 mL of phosphate buffer (pH 7.4) containing 5 mM EDTA, followed by solubilization of RP using 100 mL of ethanol. Levels of RP were then determined with HPLC. In case of pectin gel and ethanolic solution, the stability of RP was monitored after directly solubilizing RP with ethanol.
In order to evaluate the ability of PMP and PNP to release RP, nylon membrane with 0.45 µm pore size was sandwiched between donor and receiver compartments of a Franz diffusion cell. A mixture of glycerin and ethanol with a 2:8 ratio (w/w) was used as a receiver phase to ensure a sink condition. RP-loaded PMP/PNP suspended in distilled water (DW), RP-containing pectin gel (0.5 mg pectin/mL), and RP ethanolic solution were placed on the membrane, respectively. Samples (100 µL) of the receiver phase were taken at pre-determined time intervals (0, 2, 4, 6, 8, 12 and 24 h) for HPLC determination of RP.
Animal experiments were performed in accordance with an animal protocol approved by Chung-Ang University Support Center for Animal Experiments. Animals were sacrificed using bicarbonate gas, and all efforts were made to minimize suffering. Hairs on dorsal skin surface of male Wistar rats were carefully removed using an animal hair clipper and razors. The rats were the sacrificed with ether anesthesia and dorsal skin samples were then excised and subcutaneous lipid of the samples was carefully removed. The skin samples were then rinsed with PBS.
The same procedures described in the in vitro permeation study section were repeated using PMP/PNP suspensions and pectin gel along with rat skin samples obtained above instead of nylon membrane.
Following the skin permeation studies for 24 h, stratum corneum (SC) was separated by sequential adhesive-tapestripping (20 times) of the skin used for the permeation studies. RP was extracted from the tape by immersing it in 2 mL of methanol. The SC-removed skin samples were immersed in water at 60℃ for 1 min and the epidermis was then carefully separated using forceps. Each skin tissue layer was cut into small pieces using a homogenizer, immersed in methanol (5 mL) and ultra-sonicated for 30 min. The samples were filtrated using PVDF membranes with 0.45 µm pore size and the amounts of RP were assayed by HPLC.
The HPLC system consisted of Waters 2695 Separations Module and Waters 2487 dual absorbance detector (Alliance, Waters, Millford, MA). The separation of RP was achieved by Hypersil GOLD® column (4.6 mm×250 mm. 5 µm). Methanol (100%) was used as a mobile phase with a flow rate of 1.5 mL/min monitored at 320 nm.
All data are denoted as mean±SD. Statistical significance was checked by Student's t-test and p<0.05 was considered to be significantly different.
Go to : 
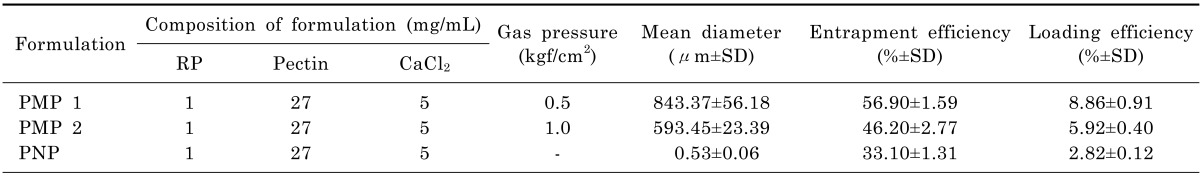
Table 1 shows compositions and characteristics of RP-loaded PMP/PNP prepared in this study. Two types of PMP (PMP 1 and PMP 2) with different mean particle sizes were produced by changing nitrogen gas pressure (0.5 and 1.0 kgf/cm2) applied to tips of syringes containing the mixture of RP ethanolic solution and pectin aqueous solution. As shown in Fig. 1A, particle sizes of PMP 1 prepared with the low gas pressure (0.5 kgf/cm2) were mainly distributed in size ranges of 710~850 and 850~1500 µm. In contrast, particle sizes of PMP 2 were primarily distributed in size ranges of 500~710 µm. Mean particle sizes of PMP 1 and 2 were 843.37±56.18 and 593.45±23.39 µm, respectively, as exhibited in Table 1. The size distribution and mean particle size of PMP could thus be controlled by changing the nitrogen gas pressure applied to the tip of syringes. In terms of PNP, the size distribution of nano-scale pectin particles loaded with RP was estimated as seen in Fig. 1B, and the mean particle size was measured to be 530±60 nm in diameter.
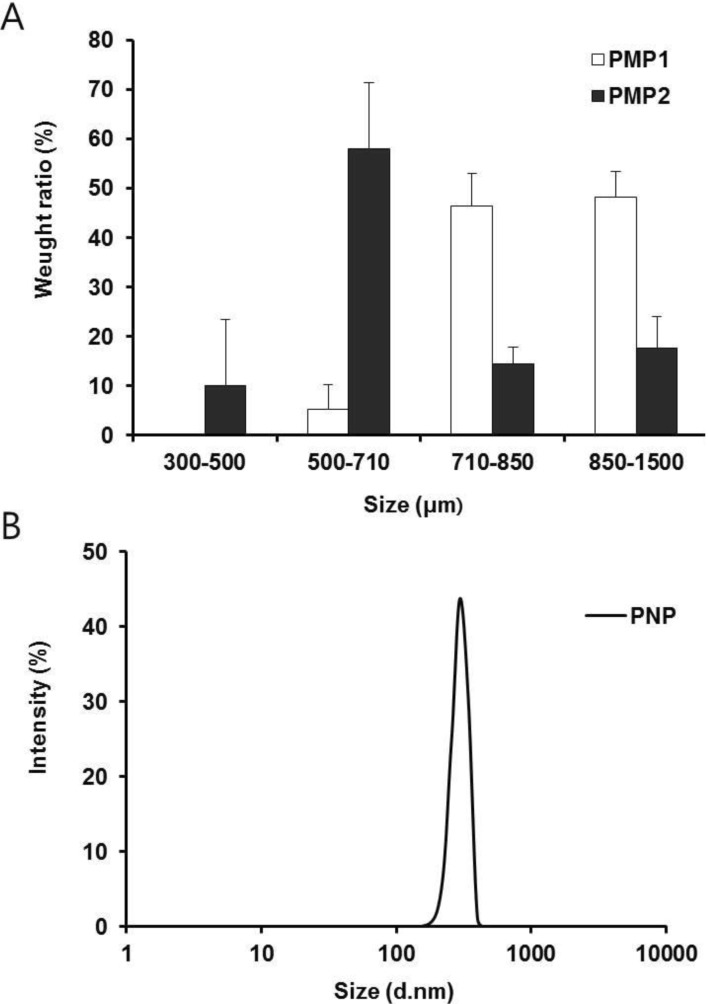
Entrapment efficiencies and loading efficiencies of PMP 1, PMP 2 and PNP were measured to be 56.90±1.59 and 8.86±0.91 (PMP 1), 46.20±2.77 and 5.92±0.40 (PMP 2), and 33.10±1.31 and 2.82±0.12%, respectively, demonstrating a gradual decrease with decreasing mean particle sizes of pectinate particles (Table 1). This result may largely be because larger surface area of smaller particles caused greater drug loss during preparation procedures of RP-loaded PMP/PNP [12]. With regard to light microscope observation of PMP 1 and PMP 2, spherical shape and smooth surface were shown (Fig. 2).
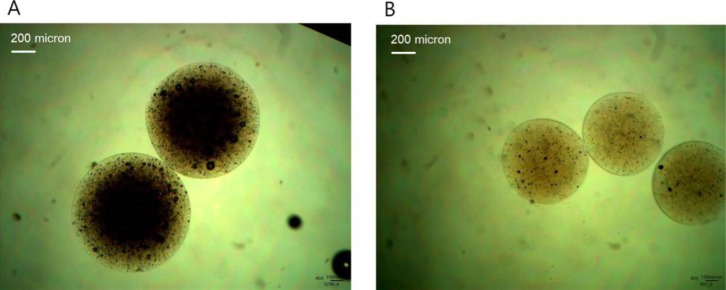
Fig. 3 illustrates DPPH radical scavenging effect of PMP and PNP as an indication of the anti-oxidative activity of RP micro- and nano-particles. The DPPH radical scavenging effect measured from PNP, PMP 1 and PMP 2 were 83.27, 63.30, and 65.44%, respectively. PNP thus exhibited the greatest anti-oxidative action, followed by PMP 2 and PMP 1, implying the DPPH radical scavenging effect increased with decreasing the particle size. The reason for this result might be because larger surface area of smaller pectinate particles facilitated the interaction between abundant hydroxyl groups of pectin and DPPH free radicals, thereby leading to further removing free radicals [13].
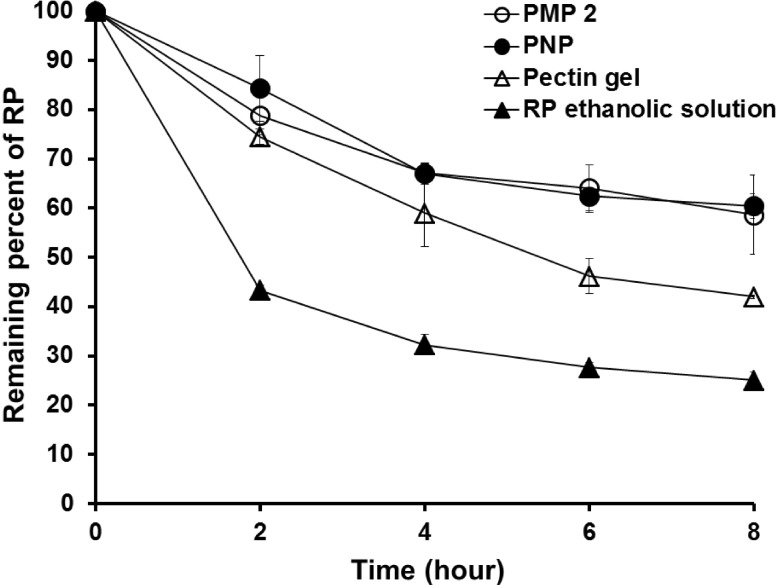
Fig. 4 shows changes in concentrations of RP in formulations tested (PMP 2, PNP, pectin gel and ethanolic solution of RP). Remaining levels of RP incorporated in PMP 2 and PNP at the end of this stability study were 58.64± 8.03 and 60.46±2.50%, respectively, which were considerably greater than those loaded in pectin gel and ethanol (42.00±0.30 and 25.04±1.70%). The stability of RP in pectin- based formulations (PMP 2, PNP and pectin gel) was therefore considerably greater than in ethanolic solution. Of the pectin-based formulations, the stabilizing effect of PMP 2 and PNP on RP was superior to pectin gel. The reason for this may be attributed to the anti-oxidative activity of pectin [8], and the protection effect of PMP 2 and PNP on RP in contact with oxidative environment. This study consequently suggests that the stability of RP could be maximized by encapsulation with polymers having anti-oxidative effects such as pectin.
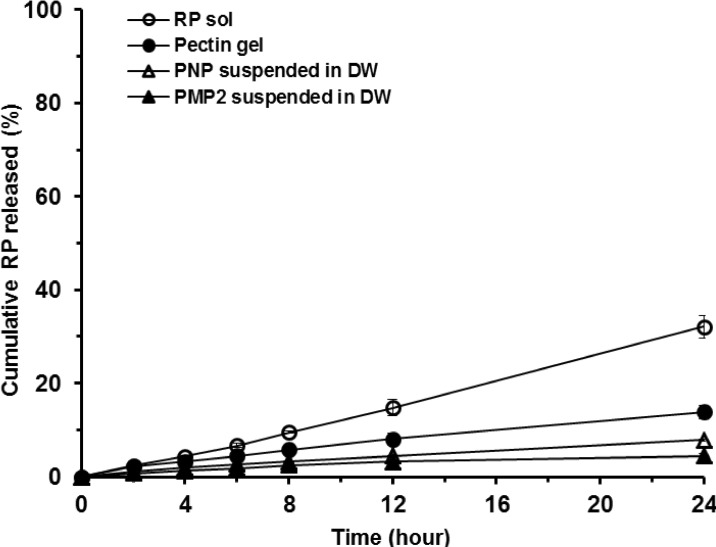
Release profiles of RP from 4 different formulations examined (PMP 2, PNP, pectin gel and ethanolic solution) during 24 h are shown in Fig. 5. The rank order of RP release and permeation rates from the test formulations was as follows: ethanolic solution > pectin gel > PNP suspension > PMP 2 suspension. The cumulative amount of RP released and permeated from PMP 2 and PNP suspensions, pectin gel, and ethanolic solution for 24 h were measured to be 4.43±0.51, 7.79±0.27, 13.87±1.40, and 32.07±2.45%, respectively. Release rates of RP from the formulations as observed from the slopes of Fig. 5 were comparatively constant, exhibiting R2 values (correlation coefficient) of 0.9214, 0.9874, 0.9880, and 0.9970, respectively. For PMP 2 and PNP, sustained release of RP compared to other formulations tested was shown largely due to cross-linked pectin with calcium ions implying the cross-linked matrices of pectinate particles could act as barriers for the release of RP and delaying the permeation of RP across the membrane. This constant and sustained release of RP from PMP and PNP would be advantageous for controlled transdermal transport of the anti-wrinkle agent RP as extended supply of RP to the skin can be expected.
To evaluate the permeation of RP through the rat skin layers, the receiver phase of Franz diffusion cells was analyzed at each time interval for 24 h. However, RP was not detected at all time points implying that RP could not permeate across the skin under these experimental conditions.
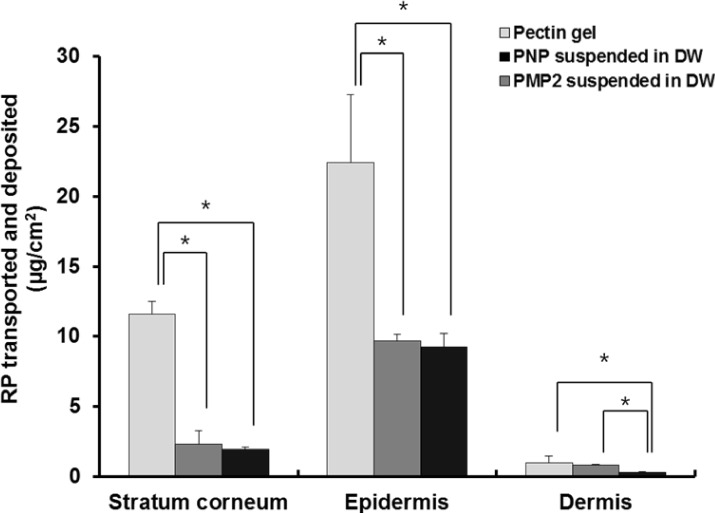
Fig. 6 illustrates the amount of RP deposited in each skin tissue (SC, epidermis, and dermis) from PMP 2 and PNP in comparison to from pectin bulk gels. Among three different vehicles examined, pectin bulk gels showed the greatest amount of RP deposited in each skin tissue, especially in SC and epidermis compared to PMP 2 and PNP. The reason for this may be because the release rate of RP from pectin gel was the fastest compared to PMP 2 and PNP. There were no significant differences of the level of RP deposited between PMP 2 and PNP suspensions in SC and epidermis but RP deposition in dermis was considerably greater from PNP than from PMP 2, probably due largely to better ability to release RP.
Nevertheless, when considering the application conditions of long-term exposure to light, oxygen, high temperature the efficacy of RP in gel formulation would greatly be limited due to its instability characteristics under these conditions [14,15]. Therefore the transdermal delivery of intact RP from gel formulation would not be promising. Ultimately, total deposited amount and the efficacy of RP might depend largely on stabilizing ability of RP provided by the delivery systems. Thus it is considerably expected that PMP 2 and PNP would be more useful as cosmetic formulations for the stabilization and efficient transport of RP because the enhanced stability of RP obtained with encapsulation technique and the ability of the sustained release would maximize the prolonged exposure of RP to the skin leading to enhanced anti-wrinkle activity of RP on the skin which is currently conducted and to be reported soon.
RP-loaded PMP and PNP prepared in this study showed considerably greater and improved anti-oxidative activity and stabilizing effect on RP among formulations examined, and these actions of the particulated vehicles were enhanced with decreasing particle sizes. In addition, PMP and PNP showed the abilities to constantly release RP and it could be permeated across the model artificial membrane and rat whole skin. The RP delivered to the skin was deposited throughout the skin layers. Based on the observation made in our work, RP loaded PMP and PNP were expected to be advantageous for improved anti-wrinkle effects. This effect would be influenced by the sizes of the particles containing RP. Highly efficient cosmeceutical products of RP would be developed when PMP and PNP are incorporated into the cosmetic bases.
Go to : 
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R&D project, Ministry of Health & Welfare, Republic of Korea (Grant No. HN10C0032). This research was supported by the Chung-Ang University Research Scholarship Grants in 2013.
Go to : 
References
1. Pena Ferreira MR, Costa PC, Bahia FM. Efficacy of antiwrinkle products in skin surface appearance: a comparative study using non-invasive methods. Skin Res Technol. 2010; 16:444–449. PMID: 20923458.

2. Hubinger JC. Determination of retinol, retinyl palmitate, and retinoic acid in consumer cosmetic products. J Cosmet Sci. 2009; 60:485–500. PMID: 19822106.

3. Boehnlein J, Sakr A, Lichtin JL, Bronaugh RL. Characterization of esterase and alcohol dehydrogenase activity in skin. Metabolism of retinyl palmitate to retinol (vitamin A) during percutaneous absorption. Pharm Res. 1994; 11:1155–1159. PMID: 7971717.
4. Jee JP, Lim SJ, Park JS, Kim CK. Stabilization of all-trans retinol by loading lipophilic antioxidants in solid lipid nanoparticles. Eur J Pharm Biopharm. 2006; 63:134–139. PMID: 16527470.

5. Tolleson WH, Cherng SH, Xia Q, Boudreau M, Yin JJ, Wamer WG, Howard PC, Yu H, Fu PP. Photodecomposition and phototoxicity of natural retinoids. Int J Environ Res Public Health. 2005; 2:147–155. PMID: 16705812.

6. Carlotti ME, Rossatto V, Gallarate M. Vitamin A and vitamin A palmitate stability over time and under UVA and UVB radiation. Int J Pharm. 2002; 240:85–94. PMID: 12062504.

7. Carlotti ME, Rossatto V, Gallarate M, Trotta M, Debernardi F. Vitamin A palmitate photostability and stability over time. J Cosmet Sci. 2004; 55:233–252. PMID: 15264052.

8. Ro J, Kim Y, Kim H, Jang SB, Lee HJ, Chakma S, Jeong JH, Lee J. Anti-oxidative activity of pectin and its stabilizing effect on retinyl palmitate. Korean J Physiol Pharmacol. 2013; 17:197–201. PMID: 23776395.

9. Suh DC, Kim Y, Kim H, Ro J, Cho SW, Yun G, Choi SU, Lee J. Enhanced In Vitro Skin Deposition Properties of Retinyl Palmitate through Its Stabilization by Pectin. Biomol Ther (Seoul). 2014; 22:73–77. PMID: 24596625.

10. Sriamornsak P, Nunthanid J. Calcium pectinate gel beads for controlled release drug delivery: I. Preparation and in vitro release studies. J Microencapsul. 1998; 160:207–212.
11. Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem. 1998; 62:1201–1204. PMID: 9692204.

12. Gray WA, Granada JF. Drug-coated balloons for the prevention of vascular restenosis. Circulation. 2010; 121:2672–2680. PMID: 20566965.

13. Alfadul SM, Elneshwy AA. Use of nanotechnology in food processing, packaging and safety review. Afr J Food Agric Nutr Dev. 2010; 10:2719–2739.
14. Carlotti ME, Ugazio E, Sapino S, Peira E, Gallarate M. Photodegradation of retinol and anti-aging effectiveness of two commercial emulsions. J Cosmet Sci. 2006; 57:261–277. PMID: 16957807.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download