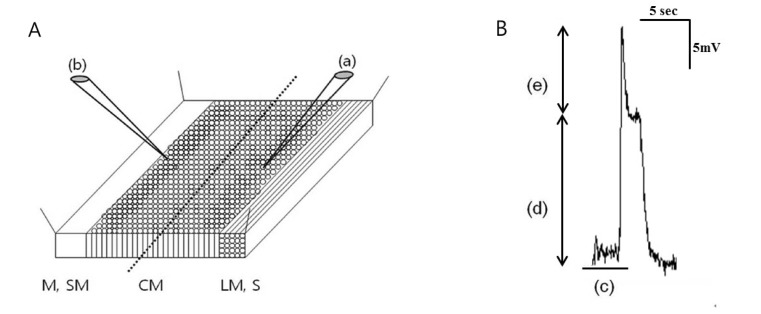
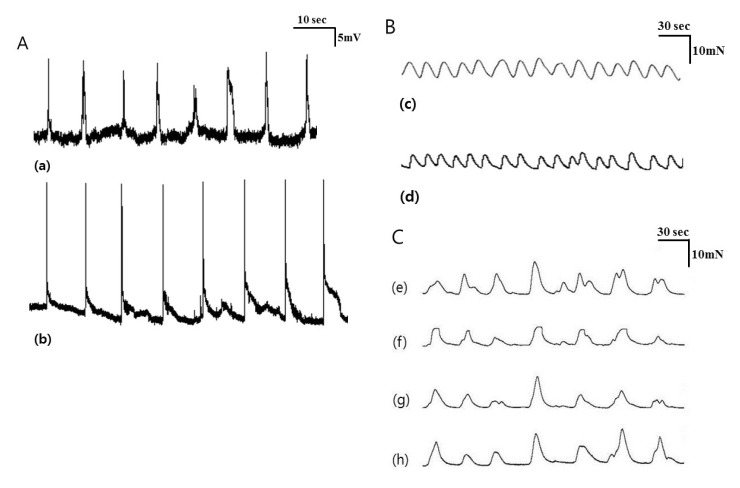
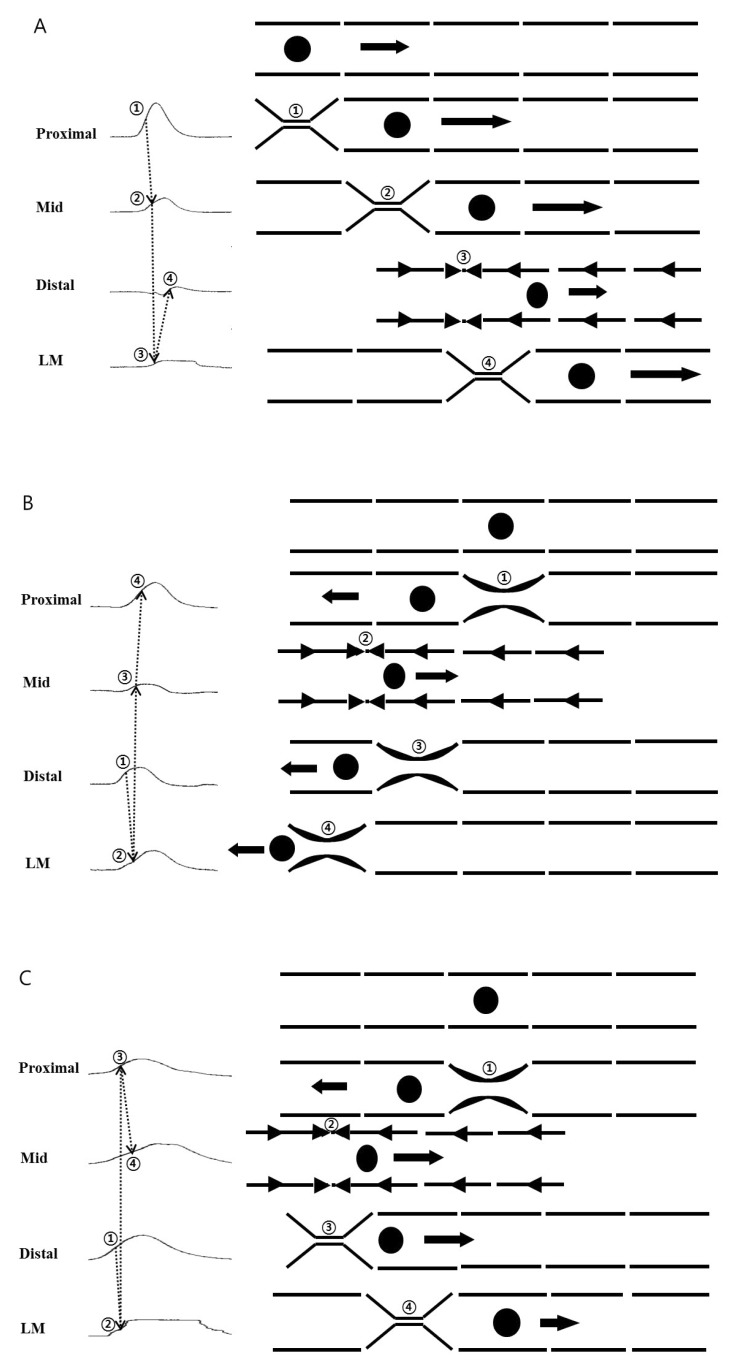
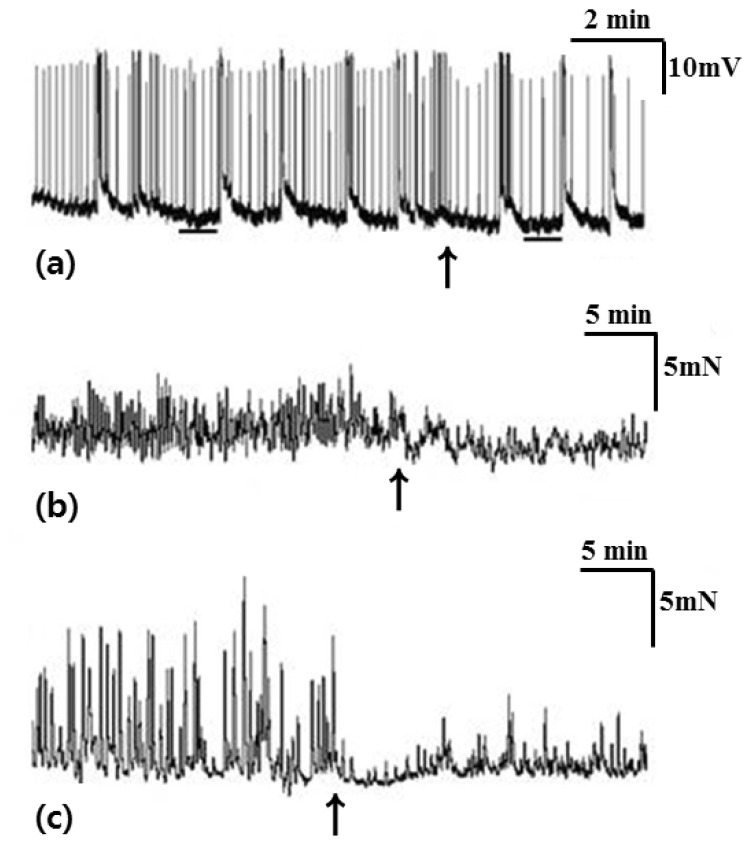
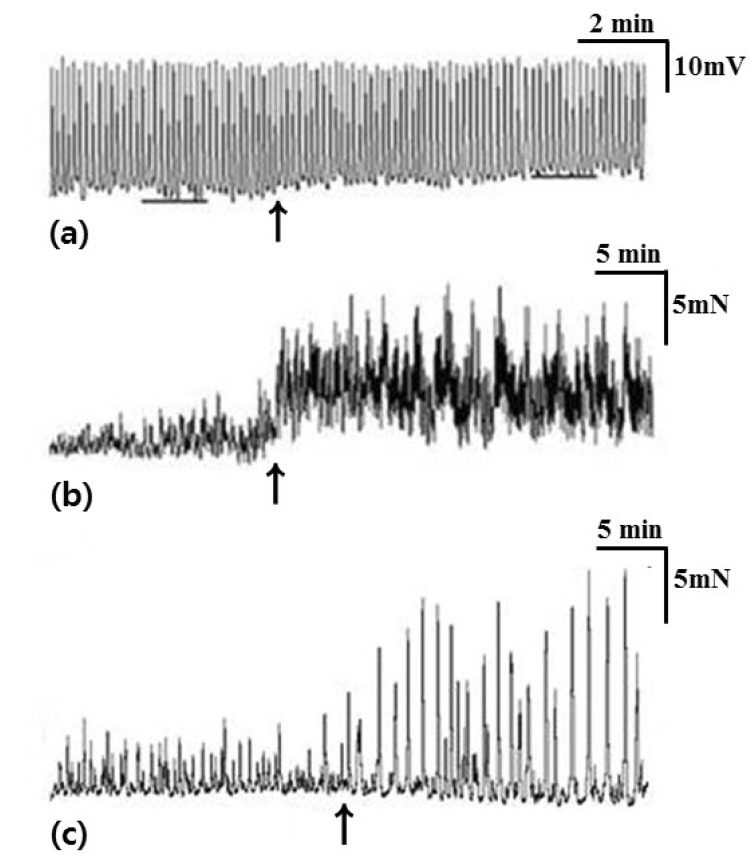
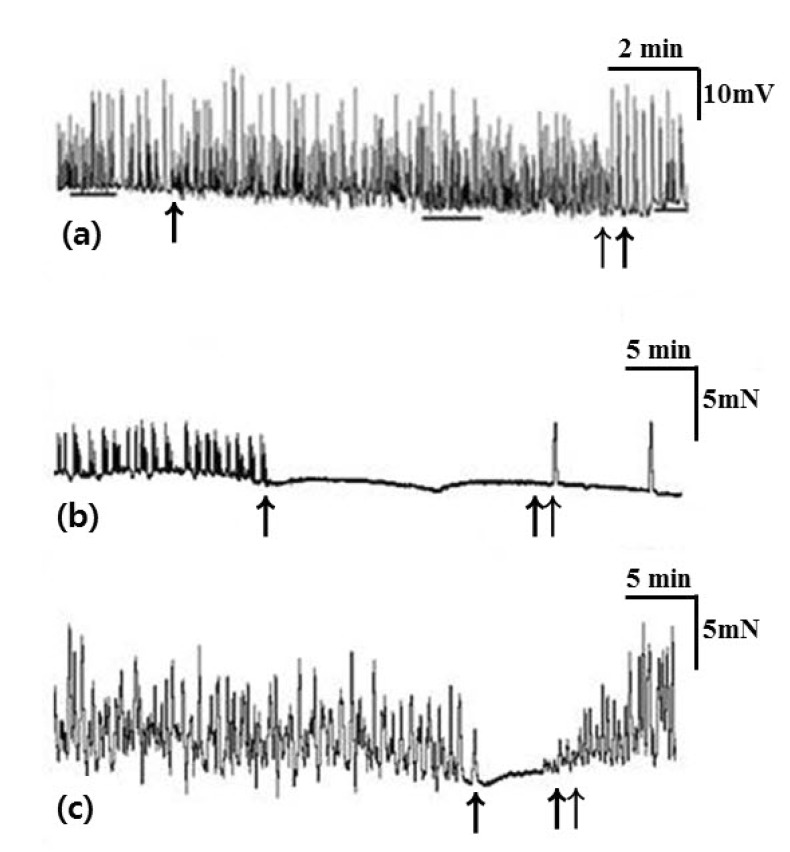
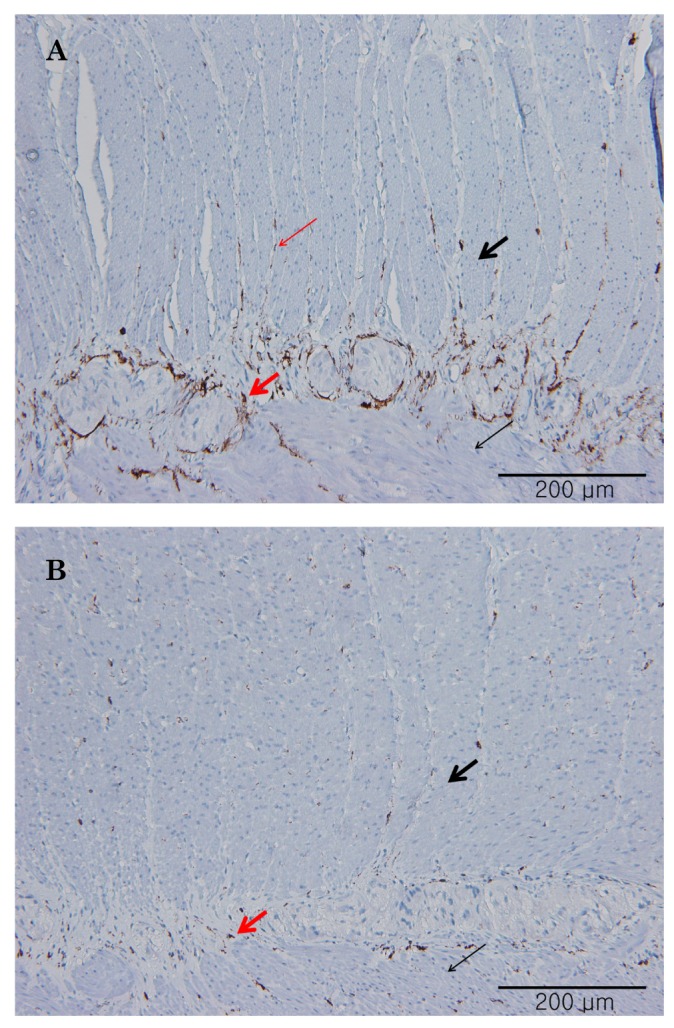
1. Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996; 111:492–515. PMID:
8690216.

2. Wingate DL. Backwards and forwards with the migrating complex. Dig Dis Sci. 1981; 26:641–666. PMID:
7018863.

3. Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982; 71:1–130. PMID:
7090872.

4. Koh SD, Sanders KM. Stretch-dependent potassium channels in murine colonic smooth muscle cells. J Physiol. 2001; 533:155–163. PMID:
11351024.

5. Spencer NJ. Control of migrating motor activity in the colon. Curr Opin Pharmacol. 2001; 1:604–610. PMID:
11757816.

6. Spencer NJ, Sanders KM, Smith TK. Migrating motor complexes do not require electrical slow waves in the mouse small intestine. J Physiol. 2003; 553:881–893. PMID:
14514874.

7. Fida R, Lyster DJ, Bywater RA, Taylor GS. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol Motil. 1997; 9:99–107. PMID:
9198085.

8. Bush TG, Spencer NJ, Watters N, Sanders KM, Smith TK. Spontaneous migrating motor complexes occur in both the terminal ileum and colon of the C57BL/6 mouse in vitro. Auton Neurosci. 2000; 84:162–168. PMID:
11111848.

9. Hara Y, Kubota M, Szurszewski JH. Electrophysiology of smooth muscle of the small intestine of some mammals. J Physiol. 1986; 372:501–520. PMID:
3723415.

10. Bauer AJ, Sarr MG, Szurszewski JH. Opioids inhibit neuromuscular transmission in circular muscle of human and baboon jejunum. Gastroenterology. 1991; 101:970–976. PMID:
1679737.

11. Stark ME, Bauer AJ, Sarr MG, Szurszewski JH. Nitric oxide mediates inhibitory nerve input in human and canine jejunum. Gastroenterology. 1993; 104:398–409. PMID:
8425682.

12. Farrelly AM, Ro S, Callaghan BP, Khoyi MA, Fleming N, Horowitz B, Sanders KM, Keef KD. Expression and function of KCNH2 (HERG) in the human jejunum. Am J Physiol Gastrointest Liver Physiol. 2003; 284:G883–G895. PMID:
12736144.

13. Ryoo SB, Oh HK, Yu SA, Moon SH, Choe EK, Oh TY, Park KJ. The effects of eupatilin (stillen®) on motility of human lower gastrointestinal tracts. Korean J Physiol Pharmacol. 2014; 18:383–390. PMID:
25352757.

14. Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. The mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterology. 2007; 132:1852–1865. PMID:
17484879.

15. Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. Septal interstitial cells of Cajal conduct pacemaker activity to excite muscle bundles in human jejunum. Gastroenterology. 2007; 133:907–917. PMID:
17678922.

16. Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility--insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012; 9:633–645. PMID:
22965426.

17. Lee HK, Sanders KM. Comparison of ionic currents from interstitial cells and smooth muscle cells of canine colon. J Physiol. 1993; 460:135–152. PMID:
8387582.

18. Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000; 525 Pt 2:355–361. PMID:
10835039.
19. Kim YC, Koh SD, Sanders KM. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol. 2002; 541:797–810. PMID:
12068041.
20. Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, Sanders KM. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol. 2006; 290:C1411–C1427. PMID:
16381798.

21. Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003; 553:803–818. PMID:
14565995.

22. Koh SD, Ward SM, Sanders KM. Ionic conductances regulating the excitability of colonic smooth muscles. Neurogastroenterol Motil. 2012; 24:705–718. PMID:
22726670.

23. Hennig GW, Hirst GD, Park KJ, Smith CB, Sanders KM, Ward SM, Smith TK. Propagation of pacemaker activity in the guineapig antrum. J Physiol. 2004; 556:585–599. PMID:
14754999.

24. Ward SM, Sanders KM. Pacemaker activity in septal structures of canine colonic circular muscle. Am J Physiol. 1990; 259:G264–G273. PMID:
2382725.

25. Horiguchi K, Semple GS, Sanders KM, Ward SM. Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum. J Physiol. 2001; 537:237–250. PMID:
11711577.

26. Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006; 68:307–343. PMID:
16460275.

27. Hara Y, Szurszewski JH. Effect of potassium and acetylcholine on canine intestinal smooth muscle. J Physiol. 1986; 372:521–537. PMID:
3723417.

28. Iino S, Horiguchi K. Interstitial cells of cajal are involved in neurotransmission in the gastrointestinal tract. Acta Histochem Cytochem. 2006; 39:145–153. PMID:
17327901.

29. Stark ME, Bauer AJ, Szurszewski JH. Effect of nitric oxide on circular muscle of the canine small intestine. J Physiol. 1991; 444:743–761. PMID:
1688034.

30. Boeckxstaens GE, Pelckmans PA, Bult H, De Man JG, Herman AG, Van Maercke YM. Non-adrenergic non-cholinergic relaxation mediated by nitric oxide in the canine ileocolonic junction. Eur J Pharmacol. 1990; 190:239–246. PMID:
1981752.

31. Park KJ, Baker SA, Cho SY, Sanders KM, Koh SD. Sulfurcontaining amino acids block stretch-dependent K
+ channels and nitrergic responses in the murine colon. Br J Pharmacol. 2005; 144:1126–1137. PMID:
15700022.
32. Powell AK, Fida R, Bywater RA. Motility in the isolated mouse colon: migrating motor complexes, myoelectric complexes and pressure waves. Neurogastroenterol Motil. 2003; 15:257–266. PMID:
12787335.

33. Huizinga JD, Stern HS, Chow E, Diamant NE, El-Sharkawy TY. Electrophysiologic control of motility in the human colon. Gastroenterology. 1985; 88:500–511. PMID:
3965340.

34. Rae MG, Fleming N, McGregor DB, Sanders KM, Keef KD. Control of motility patterns in the human colonic circular muscle layer by pacemaker activity. J Physiol. 1998; 510:309–320. PMID:
9625887.

35. Smith TK, Reed JB, Sanders KM. Interaction of two electrical pacemakers in muscularis of canine proximal colon. Am J Physiol. 1987; 252:C290–C299. PMID:
3826358.

36. Choe EK, Moon JS, Moon SB, So IS, Park KJ. Electromechanical characteristics of the human colon in vitro: is there any difference between the right and left colon? Int J Colorectal Dis. 2010; 25:1117–1126. PMID:
20544209.

37. Huizinga JD, Thuneberg L, Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal motor disorders. Trends Pharmacol Sci. 1997; 18:393–403. PMID:
9357324.