Abstract
To see the inhibitory mechanism of gentamicin in response to electrical field stimulation (EFS) using the rat bladder smooth muscle, atropine or guanethidine was treated but had no effect. Methylsergide, a non-selective 5-HT1, 5-HT2 receptor antagonist was also treated but had on effect. Kinase inhibitors, such as chelerythrine (PKC inhibitor), ML-9 (MLCK inhibitor), or Y27632 (rho kinase inhibitor) were pretreated before gentamicin treatment, but did not have effect. For U73122, a phospholipase C (PLC) inhibitor however, the inhibitory effect to gentamicin was significantly attenuated in all frequencies given by the EFS. Therefore gentamicin induced inhibitory effect on EFS response in rat bladder smooth muscle was not mediated by the activation of adrenergic, cholinergic, or serotonergic receptor. The inhibition of gentamicin might be mediated through the PLC dependent pathway, but not through the PKC, MLCK or rho kinase dependent pathway.
Urinary bladder is an elastic organ where the urine is stored and excreted by the kidneys before disposal by urination [1]. The detrusor muscle is made of smooth muscle fibers arranged in spiral, longitudinal, and circular bundles [23]. It plays a crucial role in micturition and is found in the wall of the bladder, and remains relaxed to allow the bladder to store urine, and contracts to release urine [4]. The parasympathetic nervous system is responsible for maintaining the bladder function by signaling the detrusor muscle to contract, eventually excreting the urine through the urethra by micturition [5]. The M3 receptors are responsible for the direct contraction of the bladder, whereas an indirect re-contraction is mediated by M2 receptors when a reduction in adenylyl cyclase activity reverses the relaxation induced by β-adrenoceptor stimulation. Muscarinic receptors are also located prejunctionally in the bladder on cholinergic and adrenergic nerve terminals. In the nerve terminals, M1-receptors facilitate the transmitter release and M2 or M4 receptors inhibit the transmitter release [6].
Antibiotics are agents that inhibit bacterial growth or kills bacteria. It is a substance that is antagonistic to the growth of other microorganisms. But recently, the term has become broader including antimicrobial, anti-fungal, and other compounds [7]. The antibiotic compounds can be classified on the basis of chemical or biosynthetic origin. Among the major subdivision of the antibiotics, aminoglycoside, macrolide, polymyxin, lincomycin are known to have a potential of having neuromuscular blocking effect [8]. Also, if in combination with neuromuscular blocking drugs, it may prolong the action of non-depolarizing muscle relaxants and anesthetics by drug interactions [9]. They interact with muscle relaxants primarily by acting pre-synaptically to inhibit acetylcholine release [10]. Yet, the blockade of neuromuscular function is partly understood [11]. The importance of antibiotic induced neuromuscular blockade is well known both clinically and experimentally in animals [12]. Numerous studies of aminoglycoside antibiotics proposed the predominant mechanism is a presynaptic depression of evoked transmitter release [13]. Because the neuromuscular depressant actions of the aminoglycosides are reversed by calcium it has been suggested that aminoglycosides inhibit the influx of calcium ions into nerve terminals with nerve stimulation [14].
Muscle relaxant is a drug that affects skeletal muscle function and decreases the muscle tone. It can be classified into neuromuscular blockers and spasmolytic. Neuromuscular blockers act in the neuromuscular junction, blocking the action of acetylcholine eventually leading to the relaxation of the muscle [15]. It does not have central nervous system activity. Spasmolytic, on the other hand are known as centrally acting muscle relaxant, and are used to alleviate musculoskeletal pain and spasms to reduce spasticity [16]. The specific mechanism for the muscle relaxants, however vary from drug to drug. The normal end plate function can be blocked by two mechanisms for neuromuscular blockers [17]. Non-depolarizing agents such as tubocurarine, blocks the acetylcholine from binding to nicotinic receptors. Succinylcholine, on the other hand, are nicotinic receptor agonists that mimic acetylcholine, therefore blocking muscle contraction by depolarizing to an extent that the receptor is desensitized and can no longer initiate action potential and cause muscle contraction. Pancuronium also has a structural similarity to acetylcholine, therefore acting as an endogenous ligand [18]. Agents such as papaverine, is a spasmolytic that is often used as smooth muscle relaxants [19]. It inhibits phosphodiesterase to increase intracellular cyclic AMP and phosphodiesterase inhibitors [20].
Among the antibiotics, aminoglycoside antibiotics interact with muscle relaxants or anesthetics by acting presynaptically to inhibit acetylcholine release. But aminoglycoside antibiotics also have a strong effect on relaxing the smooth muscle independently. There are hypothesis on the mechanism of how the aminoglycoside antibiotics take action, but are yet still unknown. To find out the action on the smooth muscle, comparisons of aminoglycoside antibiotics were made with agonists such as skeletal and smooth muscle relaxants and inhibitory neurotransmitters.
The aim of this study is to examine and clarify the mechanism of the smooth muscle relaxation induced by gentamicin in response to on electrical field stimulation (EFS) response in rat bladder smooth muscle.
Tissues were maintained in Kreb's buffer solution including 116.6 mM NaCl, 21.9 mM NaHCO3, 1.2 mM NaH2PO4, 3.4 mM KCl, 2.5 mM CaCl2, 5.4 mM glucose and 1.2 mM MgCl2. The solutions were prepared on the day of experiment. Doses of all compounds are reported in molar concentrations and refer to their final concentration in the organ bath.
Gentamicin sulfate salt, U73122, ML-9, Y27632, chelerythrine, and methylsergide maleate salt, were purchased from Sigma Chemical Co. (St Louis, MO). Atropine sulfate was from Merck (Whitehouse Station, NJ).
Male Sprague-Dawley rats weighting between 180 and 200 g were supplied by Samtako Bio (Osan, Korea). The animals were group-housed in cages with wire-net floors in a room controlled for temperature (24~25℃) and humidity (70~75%) and were fed a normal laboratory diet (Samtako Bio). Rats were fasted for 24 hr prior to experiment, but were allowed free access to tap water throughout. All animals were kept in raised mesh-bottom cages to prevent coprophagy. The experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Institute for Molecules-Based New Drug Development in Chung-Ang University of Korea.
Transversely oriented muscle strips measuring 2 mm wide and 7 mm long were taken from the rat bladder. The strips were then cut into 2~3 minor strips, and silk ligatures were tied at both ends. The muscle strips were mounted in separate 1 ml muscle chambers. One wire was fixed to the bottom of the muscle chamber, while the other was attached to a force transducer (FT03 Grass Instruments Co., Quincy, MA). Changes in isometric force were recorded on a polygraph (Grass model 79). They were initially stretched to 1 g to bring them to near conditions of optimal force development and were equilibrated for 90 minutes while continuously being perfused with oxygenated Kreb's buffer. During this time, tension in the muscle strips decreased rapidly and stabilized somewhere around 0.5 g, but varied by individual difference. The solution was equilibrated and maintained with a gas mixture containing 95% O2 and 5% CO2 at pH 7.4 and 37℃ throughout the study.
Concentration-response curve to gentamicin was established by increasing the concentration of the drug added to the organ bath with a 30 min contact time. For each dose, EFS of 1~8 Hz was given. To evaluate the effects of various antagonists, the tissue strips were exposed to the antagonists for 30 min and then administrated with antibiotics. The effects of gentamicin-induced relaxation in the absence and the presence of antagonists such as atropine, guanethidine, methylsergide, U73122, chelerythrine, or ML-9 was then determined by giving EFS. Muscle trips were equilibrated for 30min after washing with Kreb's solution for five times between each part within the sets of experiments. All drugs were added to the organ bath in volumes not exceeding 100 µl (10% organ bath volume).
The strips were stimulated with pulse trains of 40 V in amplitude and 10 seconds in duration, with pulse duration of 1 milliseconds at a frequency of 1~8 Hz using a stimulator (model S 88; Grass Instruments) through platinum wire electrodes placed longitudinally on either side of the strips. After a stable resting tone of muscle strips was obtained, frequency-response relationship (1~8 Hz) were constructed and the strips were washed three times and allowed to equilibrate for 30 min after EFS to permit the strips to recover completely from the stimulated responses to EFS.
The amplitude of response induced by EFS was expressed as percentage change of control. Results are expressed as means±S.E. of different experiments, with n representing the number of different animals used. Statistical significance of differences between data was determined using the two-tailed Student's t-test for paired observations or one-way analysis of variance, as appropriate. Differences were considered to be statistically significant when p<0.05, p<0.01, or p<0.001 versus Control. Doses of all drugs are reported in molar concentrations and refer to their final bath concentration.
The muscle strips isolated from the rat bladder did not display a spontaneous phasic activity, and was rather tranquil. The strips were stimulated with pulse trains of 40 V in amplitude and 10 seconds in duration, with pulse duration of 1 millisecond at the frequency at 1, 2, 4, 6, 8 Hz respectively. To test the effect of gentamicin on bladder smooth muscle, gentamicin 0.1 mM was treated 5 min before the experiment. The contractile response to EFS was decreased after treatment with gentamicin (Fig. 1A). To see the dose dependent effect of gentamicin in detail, middle doses such as 3 µM, 10 µM and 30 µM were also measured (Fig. 1B). The tone change was measured by the difference of the increased tone to the basal tone. As the dosage and frequency of EFS increased, the relaxing effect of gentamicin increased dependently. A significant decrease in the tone change appeared only in the highest dose, 0.1 mM at all frequencies measured.
Fig. 2 showed the effect of receptor blockers on gentamicin induced smooth muscle relaxation. To test whether cholinergic, adrenergic or serotonergic effect is implicated in the inhibitory effect of gentamicin on muscle relaxation, atropine (0.1 mM), guanethidine (0.1 mM) and methylsergide (10 µM) was pretreated 30 min before the experiment. Atropine is a central and peripheral muscarinic cholinergic receptors antagonist, and guanethidine is an antihypertensive agent that inhibits selective transmission in post-ganglionic adrenergic nerves, and methylsergide is a non-selective 5-HT1, 5-HT2 receptor antagonist. Atropine, guanethidine and methylsergide did not affect the gentamicin-induced inhibitory effect on muscle relaxation at different frequencies. Therefore gentamicin induced muscle relaxation on EFS response in rat bladder smooth muscle is not mediated by the activation of adrenergic, cholinergic and serotonergic receptor.
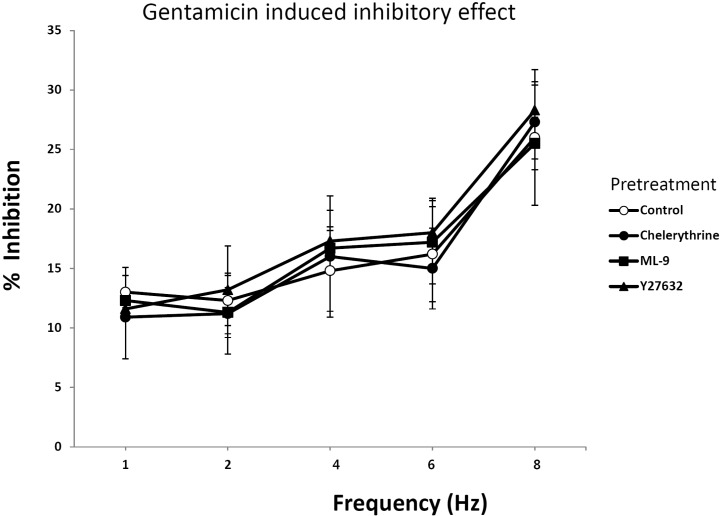
Fig. 3 showed the effect of different types of kinase inhibitors on gentamicin induced smooth muscle relaxation. Chelerythrine is a PKC inhibitor that is a competitive inhibitor on the substrate but a non-competitive inhibitor with respect to ATP. ML-9 is a myosin light chain kinase inhibitor that inhibits contraction by the blocking the myosin light chain phosphorylation. ML-9 is a myosin light chain kinase inhibitor that inhibits contraction by the blocking the myosin light chain phosphorylation. There was no significant effect of chelerythrine, ML-9 or Y27632 in inhibition of muscle relaxation induced by gentamicin at different frequencies. These data suggested that gentamicin was not related to the PKC, MLCK or Rho kinase dependent pathway.
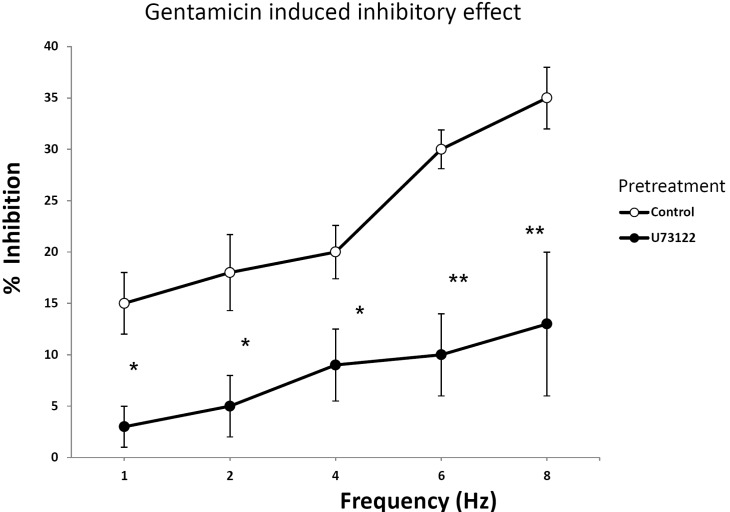
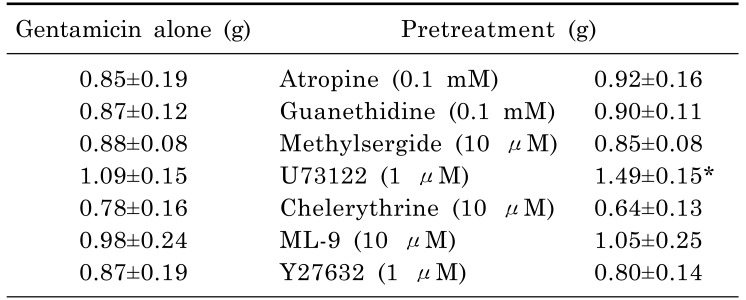
Fig. 4 showed the effect of U73122 (1 µM) on gentamicin induced smooth muscle relaxation. The effect of U73122 on gentamicin was significant at each frequency given by EFS as shown in the figure. For each frequency, U73122 decreased the EFS induced tone changes of rat bladder smooth muscle. Because the tone was decreased significantly when compared to that of the control, this data suggested that U73122 had a potential to block the action of gentamicin. Overall, the effect of gentamicin can be antagonized by blocking the PLC pathway (Table 1). The contraction was calculated as the data of 6 Hz that U73122 antagonized the inhibitory effect of gentamicin, but other antagonists did not.
Aminoglycoside antibiotics such as gentamicin, streptomycin, neomycin, and kanamycin have a strong effect on smooth muscle relaxation [212223]. Although these antibiotics are well known for its drug interactions with muscle relaxants and anesthetics, the antibiotics also have a strong muscle relaxing effect independently [2224252627]. The effect of aminoglycoside antibiotics on muscle relaxation is comparable to that of the muscle relaxants at high doses. The specific mechanism yet, remains unknown and is said to be arisen from several effects. By the inhibition of PLC, protection of calcium extrusion mechanisms and the interference with the process of calcium accumulation is the mostly well-known hypothesis.
To furthermore investigate the characteristics of the relaxing effect of antibiotics, comparisons to muscle relaxants were made and various antagonists were treated to search for the mechanism of action. First, to determine the properties of the most well-known aminoglycosides, neomycin, gentamicin, streptomycin, and kanamycin were treated cumulatively to the bladder smooth muscle under EFS. The effects of aminoglycoside antibiotics were observed at the presence of EFS to evoke neuronally mediated smooth muscle responses. The order of potency of the four antibiotics used in this study is neomycin > gentamicin > streptomycin > kanamycin [22]. In this study, all four antibiotics displayed dose dependent muscle relaxation in the presence of EFS. Furthermore, for gentamicin and neomycin, the relaxing effect was significant in every frequency given by EFS. But because gentamicin was the most effective among the four aminoglycoside antibiotics on muscle relaxation, it was used as control.
First, to see the effect of skeletal muscle relaxants on the rat bladder smooth muscle, it was treated with baclofen, tubocurarine, pancuronium and succinylcholine. Then, the tone was measured on EFS induced contraction. Baclofen is a derivative of GABA, an agonist for GABAB receptors, and is used to treat spasticity by reducing spastically increased muscle tone [28].
To determine whether the action is related with cholinergic or adrenergic mechanism was pretreated as antagonists before gentamicin [293031]. Atropine is a competitive antagonist for the muscarinic acetylcholine receptor, and guanethidine is an antihypertensive drug that reduces the release of catecholamines [3233]. The muscle strip did not have any significant result when it was pretreated with atropine and guanethidine before gentamicin was administered cumulatively. Then methylsergide, a non-selective serotonin receptor antagonist, was pretreated as antagonist but did not have any inhibitory effect on gentamicin as well. Thus, it suggests that gentamicin is not mediated by the activation of adrenergic, cholinergic, and serotonergic receptor.
Next, to find a pathway in which gentamicin takes action, inhibitors of specific kinase or phospholipase dependent pathway were treated. First, U73122, a PLC inhibitor, had a significant effect on reducing the relaxing effect of gentamicin. When compared to the control, the tone change was significant in every frequency given by EFS. For chelerythrine, a PKC inhibitor, there were not any significant results as well. ML-9, is a MLCK inhibitor that inhibits contraction by the blocking the myosin light chain phosphorylation [3435], and Y27632, is a rho kinase inhibitor [36373839]. ML-9 or Y27632, however, did not have any effect on inhibitory activity of gentamicin.
It can be concluded that gentamicin induced inhibition on EFS response in rat bladder smooth muscle is not mediated by the activation of adrenergic, cholinergic, and serotonergic receptor. Also, the effect of gentamicin on EFS response in rat bladder smooth muscle was mediated through the PLC pathway but not through the PKC, MLCK and rho kinase dependent pathway. However, further investigation is required to clarify the mechanism in-depth of how the aminoglycoside antibiotics take action.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2011-0012139).
References
1. Todd JK, Mack AJ. A study of human bladder detrusor muscle. Br J Urol. 1969; 41:448–454. PMID: 5808730.

2. Persson K, Svane D, Glavind B, Uvelius B, Forman A, Andersson KE. Effects of ovariectomy on mechanical properties and collagen content in rabbit lower urinary tract smooth muscle. Scand J Urol Nephrol. 1996; 30:7–14. PMID: 8727858.

3. Wakabayashi Y, Makiura Y, Tomoyoshi T, Kitahama K, Geffard M, Maeda T. Adrenergic innervation of the urinary bladder body in the cat with special reference to structure of the detrusor muscle: an immunohistochemical study of noradrenaline and its synthesizing enzymes. Arch Histol Cytol. 1994; 57:277–289. PMID: 7818951.

4. de Groat WC, Booth AM. Inhibition and facilitation in parasympathetic ganglia of the urinary bladder. Fed Proc. 1980; 39:2990–2996. PMID: 6106562.
5. Raezer DM, Wein AJ, Jacobowitz D, Corriere JN Jr. Autonomic innervation of canine urinary bladder. Cholinergic and adrenergic contributions and interaction of sympathetic and parasympathetic nervous systems in bladder function. Urology. 1973; 2:211–221. PMID: 4583816.

6. Chess-Williams R. Muscarinic receptors of the urinary bladder: detrusor, urothelial and prejunctional. Auton Autacoid Pharmacol. 2002; 22:133–145. PMID: 12452898.

7. Doan TL, Fung HB, Mehta D, Riska PF. Tigecycline: a glycylcycline antimicrobial agent. Clin Ther. 2006; 28:1079–1106. PMID: 16982286.

8. Wright EA, McQuillen MP. Antibiotic-induced neuromuscular blockade. Ann N Y Acad Sci. 1971; 183:358–368. PMID: 4330762.

9. Karataş Y, Ergün Y, Göçmen C, Seçilmiş A, Singirik E, Dikmen A, Baysal F. Possible postsynaptic action of aminoglycosides in the frog rectus abdominis. Acta Med Okayama. 2000; 54:49–56. PMID: 10806525.
10. Burkett L, Bikhazi GB, Thomas KC Jr, Rosenthal DA, Wirta MG, Foldes FF. Mutual potentiation of the neuromuscular effects of antibiotics and relaxants. Anesth Analg. 1979; 58:107–115. PMID: 571233.

11. Paradelis AG, Triantaphyllidis C, Giala MM. Neuromuscular blocking activity of aminoglycoside antibiotics. Methods Find Exp Clin Pharmacol. 1980; 2:45–51. PMID: 6121961.

12. Pittinger C, Adamson R. Antibiotic blockade of neuromuscular function. Annu Rev Pharmacol. 1972; 12:169–184. PMID: 4261048.

13. Corrado AP, de Morais IP, Prado WA. Aminoglycoside antibiotics as a tool for the study of the biological role of calcium ions. Historical overview. Acta Physiol Pharmacol Latinoam. 1989; 39:419–430. PMID: 2520360.
14. Wright JM, Collier B. The effects of neomycin upon transmitter release and action. J Pharmacol Exp Ther. 1977; 200:576–587. PMID: 191590.
15. Smith LR, Meyer G, Lieber RL. Systems analysis of biological networks in skeletal muscle function. Wiley Interdiscip Rev Syst Biol Med. 2013; 5:55–71. PMID: 23188744.

16. Beebe FA, Barkin RL, Barkin S. A clinical and pharmacologic review of skeletal muscle relaxants for musculoskeletal conditions. Am J Ther. 2005; 12:151–171. PMID: 15767833.

17. Ellis KO, Castellion AW, Honkomp LJ, Wessels FL, Carpenter JE, Halliday RP. Dantrolene, a direct acting skeletal muscle relaxant. J Pharm Sci. 1973; 62:948–951. PMID: 4712630.

18. Töröcsik A, Oberfrank F, Sershen H, Lajtha A, Nemesy K, Vizi ES. Characterization of somatodendritic neuronal nicotinic receptors located on the myenteric plexus. Eur J Pharmacol. 1991; 202:297–302. PMID: 1748153.
19. Iguchi M, Nakajima T, Hisada T, Sugimoto T, Kurachi Y. On the mechanism of papaverine inhibition of the voltagedependent Ca++ current in isolated smooth muscle cells from the guinea pig trachea. J Pharmacol Exp Ther. 1992; 263:194–200. PMID: 1328605.
20. Santi R, Ferrari M, Contessa AR. On the mechanism of spasmolytic effect of papaverine and certain derivatives. Biochem Pharmacol. 1964; 13:153–158. PMID: 14127294.

21. Altinkurt O, Kanzik I. Inhibitory effects of streptomycin on bronchoconstrictor, hypotensive and inflammatory responses to bradykinin and histamine. Arch Int Pharmacodyn Ther. 1980; 246:277–285. PMID: 7436632.
22. Gergawy M, Vollrath B, Cook D. The mechanism by which aminoglycoside antibiotics cause vasodilation of canine cerebral arteries. Br J Pharmacol. 1998; 125:1150–1157. PMID: 9863641.

23. Hester RK. The effects of gentamicin on tension responses to norepinephrine and KCl and Ca++ binding and fluxes in canine renal vein. Arch Int Pharmacodyn Ther. 1983; 264:118–134. PMID: 6625762.
24. Mogami H, Lloyd Mills C, Gallacher DV. Phospholipase C inhibitor, U73122, releases intracellular Ca2+, potentiates Ins(1,4,5)P3-mediated Ca2+ release and directly activates ion channels in mouse pancreatic acinar cells. Biochem J. 1997; 324:645–651. PMID: 9182729.
25. Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990; 172:993–999. PMID: 2244923.

26. Eckly-Michel AE, Le Bec A, Lugnier C. Chelerythrine, a protein kinase C inhibitor, interacts with cyclic nucleotide phosphodiesterases. Eur J Pharmacol. 1997; 324:85–88. PMID: 9137917.

27. Hague BA, Martinez EA, Hartsfield SM. Effects of high-dose gentamicin sulfate on neuromuscular blockade in halothane-anesthetized horses. Am J Vet Res. 1997; 58:1324–1326. PMID: 9361900.
28. Milanov IG. Mechanisms of baclofen action on spasticity. Acta Neurol Scand. 1992; 85:305–310. PMID: 1621492.

29. Okishio Y, Niioka S, Yamaji M, Yamazaki Y, Nishio H, Takeuchi T, Hata F. Mediators of nonadrenergic, noncholinergic relaxation in Sprague Dawley rat intestine: comparison with the mediators of other strains. J Vet Med Sci. 2000; 62:821–828. PMID: 10993178.

30. Sahin AS, Atalik KE, Dogan N. Nonadrenergic, noncholinergic responses of the rabbit detrusor smooth muscle and the role of L-arginine/nitric oxide pathway in these responses. Methods Find Exp Clin Pharmacol. 2000; 22:31–35. PMID: 10791292.

31. Niioka S, Takeuchi T, Kishi M, Ishii T, Nishio H, Takewaki T, Hata F. Nonadrenergic, noncholinergic relaxation in longitudinal muscle of rat jejunum. Jpn J Pharmacol. 1997; 73:155–161. PMID: 9074949.
32. Jing L, Inoue R, Tashiro K, Takahashi S, Ito Y. Role of nitric oxide in non-adrenergic, non-cholinergic relaxation and modulation of excitatory neuroeffector transmission in the cat airway. J Physiol. 1995; 483:225–237. PMID: 7776234.

33. Lindén A, Löfdahl CG, Ullman A, Skoogh BE. Nonadrenergic, noncholinergic responses stabilize smooth muscle tone, with and without parasympathetic activation, in guinea-pig isolated airways. Eur Respir J. 1993; 6:425–433. PMID: 8472834.
34. Watanabe H, Takahashi R, Zhang XX, Kakizawa H, Hayashi H, Ohno R. Inhibition of agonist-induced Ca2+ entry in endothelial cells by myosin light-chain kinase inhibitor. Biochem Biophys Res Commun. 1996; 225:777–784. PMID: 8780689.
35. Ishikawa T, Chijiwa T, Hagiwara M, Mamiya S, Saitoh M, Hidaka H. ML-9 inhibits the vascular contraction via the inhibition of myosin light chain phosphorylation. Mol Pharmacol. 1988; 33:598–603. PMID: 3380076.
36. Chiba Y, Takeyama H, Sakai H, Misawa M. Effects of Y-27632 on acetylcholine-induced contraction of intact and permeabilized intrapulmonary bronchial smooth muscles in rats. Eur J Pharmacol. 2001; 427:77–82. PMID: 11553366.

37. Kawada N, Seki S, Kuroki T, Kaneda K. ROCK inhibitor Y-27632 attenuates stellate cell contraction and portal pressure increase induced by endothelin-1. Biochem Biophys Res Commun. 1999; 266:296–300. PMID: 10600496.

38. Thieme H, Nuskovski M, Nass JU, Pleyer U, Strauss O, Wiederholt M. Mediation of calcium-independent contraction in trabecular meshwork through protein kinase C and rho-A. Invest Ophthalmol Vis Sci. 2000; 41:4240–4246. PMID: 11095621.
39. Yoshii A, Iizuka K, Dobashi K, Horie T, Harada T, Nakazawa T, Mori M. Relaxation of contracted rabbit tracheal and human bronchial smooth muscle by Y-27632 through inhibition of Ca2+ sensitization. Am J Respir Cell Mol Biol. 1999; 20:1190–1200. PMID: 10340938.
Fig. 1
Representative tracing showing the effect of gentamycin on EFS in the rat bladder smooth muscle. Gentamicin was added cumulatively with an interval of 5 min. The tone was measured under the EFS of 1, 2, 4, 6, 8 Hz. Each point represents the mean±SE (n=8).
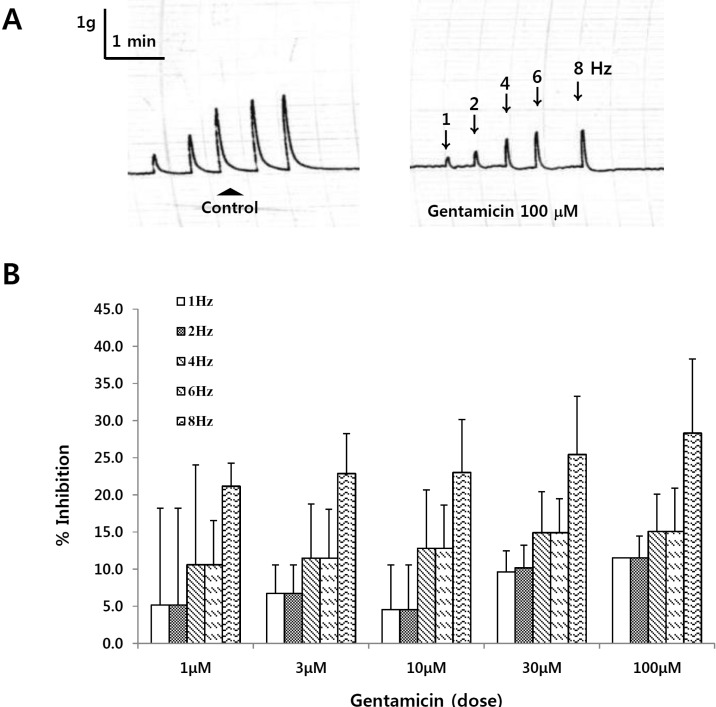
Fig. 2
Effect of atropine, guanethidine and methylsergide on EFS in rat bladder smooth muscle treated with gentamicin. Atropine (0.1 mM), guanethidine (0.1 mM) or rmethylsergide (10 µM) was treated 30 min prior to EFS stimulation. The tone was measured under the EFS of 1~8 Hz. Each point represents the mean±SE (n=8).
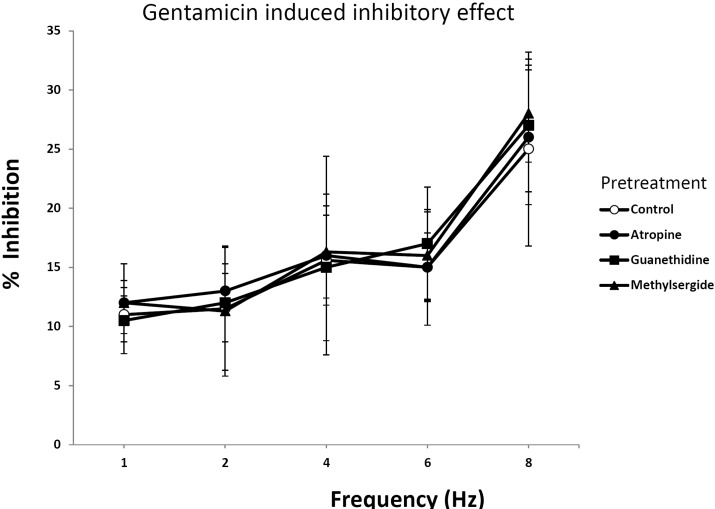
Fig. 3
Effect of chelerythrine, ML-9 and Y27632 on EFS in rat bladder smooth muscle treated with gentamicin. Chelerythrine (10 µM), ML-9 (10 µM) and Y27632 (1 µM) was treated 30 min prior to EFS stimulation. The tone was measured under the EFS of 1~8 Hz. Each point represents the mean±SE (n=8).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download