INTRODUCTION
METHODS
Isolation of EC from mouse vessels
Uptake of DiI-Ac-LDL
Western blotting
RNA isolation and RT-PCR
Flow cytometry
Immunohistochemistry
Statistical analysis
RESULTS
EC isolation, culture, and analysis
 | Fig. 1Pieces of mouse vessels explanted on/in a Matrigel support. Cells started to migrate from the edges of explants and reached confluence. (A) Aortic pieces were placed with the intima side down on Matrigel (left panel) and removed after 5~7 days (middle panel). Higher magnification images of the boxed areas, designated as a and b, are displayed in the right upper and lower sides, respectively. Cells proliferated to form a tube-like structure. (B) Schematic representations of branches of the SMA (left panel) and the CA from the circle of Willis (right panel). Branches of the SMA and the CA from the circle of Willis were explanted in Matrigel, and cells migrated from small segments of the vessels. Images were obtained by phase-contrast microscopy at 50× magnification. |
 | Fig. 2Labeling of MAEC with DiI-Ac-LDL. (A, B) Active DiI-Ac-LDL uptake was examined by flow cytometry in MAEC at passages 1-5. MAEC at passage 1 without DiI-Ac-LDL were used as a negative control. |
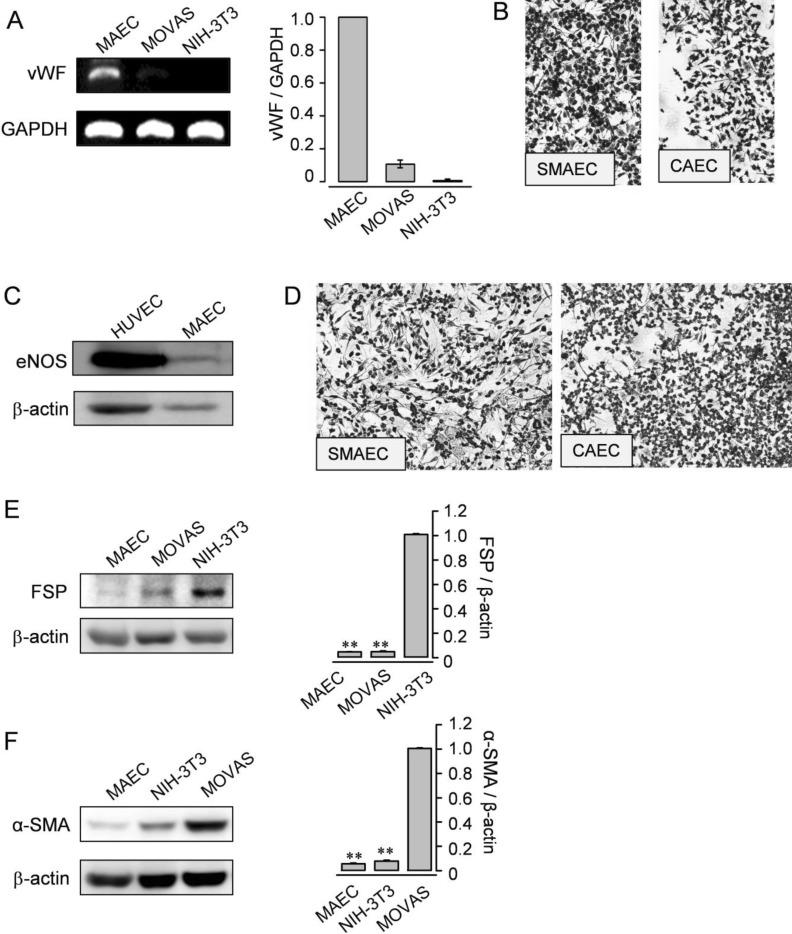 | Fig. 6Expression of typical genes and proteins from ECs. (A) RT-PCR analysis for vWF was performed (passage 5) in MAEC. SMC (MOVAS) and fibroblasts (NIH-3T3) were employed as negative controls. GAPDH was used as an internal standard. (B) Immunocytochemistry of the isolated EC from branches of the SMA or the CA for vWF. (C) Western blotting for eNOS was examined in MAEC (passage 2) and HUVEC. HUVEC were used as a positive control. (D) Immunocytochemistry of the isolated EC from branches of the SMA or the CA for eNOS. (E and F) Western blotting for FSP (E) or α-SMA (F) was examined in MAEC (passage 5), MOVAS, and NIH-3T3. NIH-3T3 or MOVAS was used as a positive control. β-actin was used as an internal standard. (A, E, and F) Results are representative of three independent experiments. The data shown are mean±SEM of three independent experiments. |
Endothelial isolation procedure
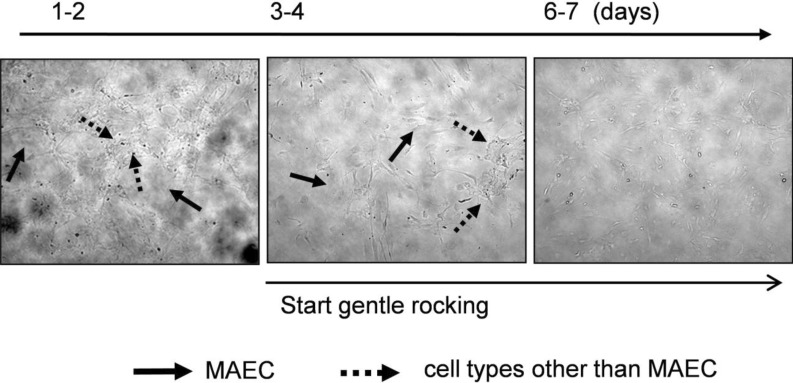 | Fig. 3Elimination of cells in the multilayer. Cells growing in the multilayer were markedly increased after passaging (left panel). When exposed to anti-fibroblast antibody and gentle rocking, cells in the multilayer gradually disappeared. Four or five days after the exposure, only cells growing in a monolayer remained. Lined arrows represent cells in a single layer, and dotted arrows are cells in a multilayer. |
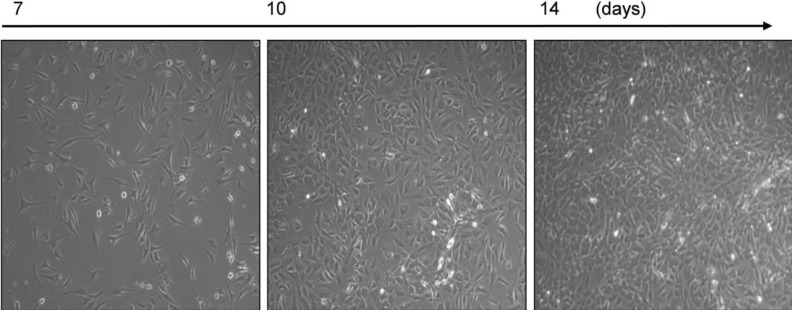 | Fig. 4Purified MAECs by elimination of cells in the multilayer. Cells from aortic explants were exposed to anti-fibroblast antibody and gentle rocking, eliminating cells in the multilayer. A confluent monolayer of MAECs was demonstrated. Note the presence of contact inhibition and cobblestone morphology in all panels. Images were obtained by phase-contrast microscopy at 100× magnification. |
 | Fig. 5Typical EC characteristics of isolated cells. (A) Cells were stained with DiI-Ac-LDL, and HUVECs were employed as a positive control (left panel). DAPI was used for nucleus staining (center panel), and merged images are displayed (right panel). DiI-Ac-LDL uptake occurred in nearly all of the cells. (B) Flow cytometry analysis was performed in MAECs (passage 4) and HUVEC. DiI-Ac-LDL-labeled ECs showed a dominant fluorescence shift compared to unlabeled negative control cells. (C) Active DiI-Ac-LDL uptake in EC isolated from the CA (CAEC, left panel) and from branches of the SMA (SMAEC, right panel). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download