Abstract
Chronic inflammation has been proposed as one of the main molecular mechanisms of aging and age-related diseases. Although evidence in humans is limited, short-term calorie restriction (CR) has been shown to have anti-inflammatory effects in aged experimental animals. We reported on the long-term treatment of daumone, a synthetic pheromone secreted by Caenorhabditis elegans in an energy deficient environment, extends the life-span and attenuates liver injury in aged mice. The present study examined whether late onset short-term treatment of daumone exerts anti-inflammatory effects in the livers of aged mice. Daumone was administered orally at doses of 2 or 20 mg/kg/day for 5 weeks to 24-month-old male C57BL/6J mice. Increased liver macrophage infiltration and gene expression of proinflammatory cytokines in aged mice were significantly attenuated by daumone treatment, suggesting that short-term oral administration of daumone may have hepatoprotective effects. Daumone also dose-dependently suppressed tumor necrosis factor-α (TNF-α)-induced nuclear factor-κB (NF-κB) phosphorylation in HepG2 cells. The present data demonstrated that short-term treatment of daumone has anti-inflammatory effects in aged mouse livers possibly through suppression of NF-κB signaling and suggest that daumone may become a lead compound targeting aging and age-associated diseases.
Finding effective anti-aging strategies has recently become one of the most active researches in the life sciences. Calorie restriction (CR) has been well established as the most effective intervention to extend the lifespan and alleviate age-related disorders in mammals, including mice and monkeys. Although CR has not been verified to extend the lifespan in humans [1,2], there is an interest developing CR mimetics with the goal of extending the lifespan in humans. Energy deficits induced by CR activates AMP-activated protein kinase (AMPK) [3], which suppresses the mammalian target of rapamycin (mTOR) which is upregulated in age-related diseases such as diabetes, obesity, and cancers [4]. Metformin, an AMPK activator, and rapamycin, an inhibitor of the raptor composed of mTOR complex 1, extends the lifespan of normal mice [5,6]. We have recently reported that synthetic daumone, a pheromone secreted by Caenorhabditis elegans in response to food deficiency, improves survival in mice [7]. Considering that daumone responds to food deprivation during the dauer stage, daumone would be proposed as a CR mimetic [8].
Aging is accompanied by the continuous status of the low grade chronic inflammation [9]. Inflammation in aging is not only the result of age-related diseases but the cause of them [10]. One of the defense mechanisms against external stress is the immune system. However, continuous overstimulation of the immune system over time result in chronic inflammation, which leads to aging [9]. Studies have found that proinflammatory cytokines are increased in the plasma of aged human and experimental animals [11]. Healthy centenarians consistently show low levels of inflammatory cytokines [12]. During aging, proinflammatory molecules are up-regulated in the liver [13,14,15], and the number and phagocytic activity of Kupffer cells, which are liver macrophages, increase with aging [16]. In our previous study, daumone given at doses that extend the lifespan attenuates age-related liver injury, including inflammation and insulin resistance, in aged mice [7].
Interestingly, short-term CR largely reproduced the effects of long-term CR on the expression of inflammatory response genes in aged mice [17]. Hepatic gene expression changes associated with inflammation are down-regulated in aged mice on short-term CR for 4 weeks. These effects of short-term CR are similar those of long-term CR for 26 months. It has also been shown that short-term CR had anti-inflammatory effects on the kidneys of aged rats [18]. Activated nuclear factor-κB (NF-κB) signaling examined by binding activity and nuclear translocation in 24-month-old rat kidneys were attenuated after 10 days of CR. These data suggest that many of anti-inflammatory effects of CR are established rapidly. The present study, thus, examined the effect of late onset short-term treatment with daumone on hepatic inflammation in aged mice. We also determined the dose-response effect of daumone on tumor necrosis factor-α (TNF-α)-induced NF-κB activation in HepG2 cells.
All chemicals were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA), and tissue culture plates from NUNC (Roskilde, Denmark) and Becton Dickinson Labware (Lincoln Park, NJ, USA), unless otherwise stated. Daumone was synthesized according to the previous report [19].
All animal experiments were conducted according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Ewha Womans University (2010-27-1). Six-month-old C57BL/6J male mice were purchased from Charles River Laboratory (Shizuoka, Japan), housed in a room maintained at 22±2℃, exposed to a 12-hour dark/12-hour light cycle, and fed a standard chow diet (PMI® Nutrition International, LLC Certified Rodent LABDIET® 5053, Purina Mills, Richmond, IN, USA) and autoclaved water ad libitum. Mice were maintained until the age of 24 months (old mice). Old mice were randomly divided into three groups; two groups received daumone in drinking water (2 mg/kg/day or 20 mg/kg/day) and the third received the same volume of untreated drinking water by oral gavage for 5 weeks. When daumone administration started, 9-week-old control mice (young mice) were purchased from Charles River Laboratory. Young mice were randomly divided into two groups; one received daumone (20 mg/kg/day) in their drinking water and the other received untreated drinking water. Each group of old mice contained 10 animals. Mice in the fed state were anesthetized with Avertin (intraperitoneal injection, i.p., 0.3 g/kg), and blood was collected. Livers were collected and immediately stored at -70℃.
HepG2 cells, human hepatoma cells, were purchased from American Type Culture Collection (Manassas, VA, USA), and maintained in Dulbecco's Modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen), 100 U/mL penicillin (Invitrogen), 100 µg/mL streptomycin (Invitrogen), and 44 mM NaHCO3 at 37℃ in humidified 5% CO2 in air. Growth arrested and synchronized cells were treated with 5 ng/mL TNF-α (R&D Systems, Minneapolis, MN, USA). The cells were pretreated with daumone 6 hours before the addition of TNF-α.
The blood HbA1c level was measured using the DCA2000 HbA1c reagent kit (SIEMENS Healthcare Diagnostics, Inc., Tarrytown, NY, USA). The blood was centrifuged at 900 g for 15 minutes at 4℃ and the supernatant was collected. The levels of plasma alanine transaminase (ALT) free fatty acids (FFA), total cholesterol, and triglycerides were measured using an EnzyChrom™ colorimetric assay kit (BioAssay Systems, Hayward, CA, USA).
Livers were fixed in 4% formalin, dehydrated, and embedded in paraffin for sectioning. Immunohistochemistry was performed with immunoperoxidase procedures and a commercially available kit (Dakocytomation, Glostrup, Denmark). Briefly, the sections were deparaffinized, and the endogenous peroxidase activity was quenched with Dako peroxidase solution. Then, the sections were incubated with Dako serum-free blocking solution prior to incubation with anti-F4/80 (1:200 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibody overnight at 4℃. The sections were subsequently incubated with the rat ABC staining kit (Santa Cruz Biotechnology). After washing with PBS, the sections were exposed to 3,3'-diaminobenzidine. Digital images were captured on a Zeiss microscope equipped with an Axio Cam HRC digital camera (Carl Zeiss, Thornwood, NY, USA), and the positive staining area in 10 fields (100× magnification) per animal was analyzed with Image-Pro Plus 4.5.1. (Media Cybernetics, Silver Springs, MD, USA).
A standard real-time qRT-PCR was performed. Total RNA was extracted from tissues using TRIzol (Invitrogen, Carlsbad, CA, USA). The mRNA levels were assessed by real-time qRT-PCR using the SYBR Green PCR Master Mix kit (Applied Biosystems, Foster City, CA, USA) with an ABI 7300 Real-time qRT-PCR thermal cycler (Applied Biosystems) as previously described [7]. The relative levels of the test genes and the internal control 18S ribosomal RNA (rRNA) were determined using a standard curve produced with the Applied Biosystems software. The primer sequences are shown in Table 1.
The protein levels in HepG2 cell homogenates were measured using a standard Western blotting protocol as previously described [7]. The protein concentrations of the samples were determined with the Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein from each sample were mixed with loading buffer and separated on SDS-PAGE gels by electrophoresis. The proteins were then transferred onto a polyvinylidene fluoride membrane (GE Healthcare BioSciences, Piscataway, NJ, USA), and the membranes were incubated overnight at 4℃ with a 1:2,000 dilution of antibodies to p-NF-κB-p65 (Ser536, Cell Signaling Technology, Danvers, MA, USA), t-NF-κB (p65, Cell Signaling Technology), p-inhibitor of NF-κBα (p-IκBα, Ser32/36, Cell Signaling Technology), and t-IκBα (Santa Cruz Biotechnology). Next, the membranes were incubated with peroxidase-conjugated secondary antibodies, and the signals were visualized with an enhanced chemiluminescence system detection reagent (GE Healthcare Bio-Sciences). Positive immunoreactive bands were quantified with a densitometer (LAS-3000, FUJIFILM, Tokyo, Japan) and normalized to the levels of total protein and β-tubulin (Santa Cruz Biotechnology).
All results are expressed as the mean±standard error (SE). Analysis of variance (ANOVA) was used to assess differences between multiple groups. If the F statistic was significant, the mean values of each group were compared with the Fisher's least significant difference method. A p value<0.05 was considered statistically significant.
Old mice received daumone for 5 weeks from the age of 24 months by oral gavage. Twenty five-month-old mice showed significantly increased body weight and liver weight compared to young mice (Table 2). The plasma ALT of old mice was twice that of young mice. Plasma lipid such as FFA and total cholesterol were significantly increased in old mice compared to young mice. Daumone administration at doses of 2 and 20 mg/kg/day effectively decreased age-associated FFA but did not affect liver weight and plasma ALT. No significant differences were observed in plasma triglycerides and HbA1c between old and young mice.
Macrophage infiltration established by F4/80 staining showed that hepatic macrophage infiltration was accelerated in old mice compared to young mice, but this was effectively reduced by the daily administration of 20 mg/kg of daumone (Fig. 1A and B). The mRNA expression of F4/80 (Emr1) was significantly increased in old mice, which was unaffected by daumone treatment for 5 weeks (Fig. 1C).
Inflammatory cytokines, including Ccl2, Icam1, Tnf, and Nos2 mRNA expression were significantly increased in the aged liver (Fig. 1D-G). Icam1 and Tnf mRNA expressions were effectively reduced by daumone administration of both 2 and 20 mg/kg/day. Ccl2 and Nos2 mRNA expressions were effectively reduced by the administration of 20 mg/kg/day of daumone.
To assess the mechanism of the anti-inflammatory effects of daumone, the levels of TNF-α-induced NF-κB and IκBα phosphorylation in HepG2 cells were measured. TNF-α significantly increased NF-κB (Fig. 2A and B) and IκBα (Fig. 2A and C) phosphorylation, both of which were significantly suppressed by 0.5 and 1 mM daumone treatment.
The present study clearly shows that short-term treatment of daumone for 5 weeks starting in 24-month-old mice effectively attenuated age-associated hepatic inflammation in a dose-dependent manner. Cell culture study suggests inhibition of NF-κB activation as a possible underlying mechanism for the anti-inflammatory effect of daumone.
The efficacy of short-term treatment of daumone on aged mice liver was smaller than but similar with the results from late onset long-term treatment of daumone which reduced the increased histological macrophage infiltration, gene expression of Ccl2, Icam1, Tnf, and Nos2, and the risk of death and hepatic inflammation in aged mice [7]. Short-term treatment of daumone did not attenuate Emr1 (F4/80) gene expression in the liver of aged mice, unlike that of long-term daumone treatment. It will advance our understanding on molecular mechanism of daumone to examine systemic as well as hepatic inflammatory gene expression signature. The anti-inflammatory effects of daily treatment with 20 mg/kg of daumone were generally more effective than 2 mg/kg/day. Since daumone treatment up to 20 mg/kg/day for 5 weeks did not show apparent toxicity or changes in food intake, body weight change, liver function (Table 1), and behavior (data not shown), additional studies are needed to investigate whether long-term treatment with 20 mg/kg/day of daumone could more effectively improve survival and hepatic inflammation than 2 mg/kg/day [7].
Our results are consistent with previous reports demonstrating anti-inflammatory effect of short-term CR. Although it is limited, late onset CR has been suggested as a potential application to intervene and reverse age-associated organ damage [17,20,21,22]. Even a short duration of CR for 10 days has anti-inflammatory effects on 24-month-old rat kidneys [18], and CR for 4 weeks as well as lifelong CR reversed the majority of age-associated gene expression in 34-month-old mice livers [17].
Hepatic injury estimated by increased ALT was apparent in 25-month-old mice, and was not altered by short-term daumone treatment, which differs from the results of long-term daumone treatment [7]. Three months of CR reduced senescence associated with β-galactosidase (SA-β-gal) activity and improved telomere maintenance in 14-month-old mice livers [21], suggesting that the anti-inflammatory effect may be the early therapeutic benefit of CR mimetics or anti-aging agents. Experiments like these should be utilized to quickly identify effective CR mimetics or anti-aging agents.
The anti-inflammatory effects of daumone may result from inhibition of NF-κB. Lifelong CR attenuates nuclear NF-κB and cytosolic IκBα phosphorylation in 24-month-old mice livers [15]. Similarly, long-term treatment of daumone reduces cytosolic IκBα phosphorylation in aged mice livers [7]. In the present study, increased IκBα and NF-κ B-p65 phosphorylation in TNF-α-treated HepG2 cells were concentration-dependently reduced by daumone treatment. It remains to be determined whether daumone affects macrophage NF-κB activation. NF-κB activation in macrophages leads to upregulate inflammatory cytokines including TNFα [23,24]. However, inhibition of macrophage NF-κB through macrophage-restricted deletion of IκB kinase 2 increases atherosclerosis [25].
The present data demonstrated for the first time that late onset short-term treatment of daumone, a possible CR mimetic, has protective effects on liver inflammation through the suppression of NF-κB activation, and suggests that daumone may become a lead compound in the treatment of age and age-related diseases.
ACKNOWLEDGMENT
This study was supported by NRF-2012R1A2A1A03006092 from an NRF grant funded by the Korean Government (MEST).
References
1. Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001; 414:412. PMID: 11719795.

2. Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010; 328:321–326. PMID: 20395504.

3. Cantó C, Auwerx J. Calorie restriction: is AMPK a key sensor and effector? Physiology (Bethesda). 2011; 26:214–224. PMID: 21841070.

4. Menzies KJ, Hood DA. The role of SirT1 in muscle mitochondrial turnover. Mitochondrion. 2012; 12:5–13. PMID: 21406254.

5. Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009; 460:392–395. PMID: 19587680.

6. Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013; 4:2192. PMID: 23900241.

7. Park JH, Chung HY, Kim M, Lee JH, Jung M, Ha H. Daumone fed late in life improves survival and reduces hepatic inflammation and fibrosis in mice. Aging Cell. 2014; 13:709–718. PMID: 24796965.

8. Hu PJ. Dauer. WormBook: The Online Review of C. elegans Biology. In : Riddle DL, editor. WormBook. United States: The C. elegans Research Community;2007. p. 1–19.
9. Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007; 128:92–105. PMID: 17116321.

10. Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010; 16:238–246. PMID: 20444648.

11. Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009; 8:18–30. PMID: 18692159.

12. Franceschi C, Bonafè M. Centenarians as a model for healthy aging. Biochem Soc Trans. 2003; 31:457–461. PMID: 12653662.

13. Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006; 8:572–581. PMID: 16677101.

14. Chung HY, Kim HJ, Kim JW, Yu BP. The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann N Y Acad Sci. 2001; 928:327–335. PMID: 11795524.
15. Seo AY, Hofer T, Sung B, Judge S, Chung HY, Leeuwenburgh C. Hepatic oxidative stress during aging: effects of 8% long-term calorie restriction and lifelong exercise. Antioxid Redox Signal. 2006; 8:529–538. PMID: 16677097.

16. Hilmer SN, Cogger VC, Le Couteur DG. Basal activity of Kupffer cells increases with old age. J Gerontol A Biol Sci Med Sci. 2007; 62:973–978. PMID: 17895435.

17. Cao SX, Dhahbi JM, Mote PL, Spindler SR. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci U S A. 2001; 98:10630–10635. PMID: 11535822.

18. Jung KJ, Lee EK, Kim JY, Zou Y, Sung B, Heo HS, Kim MK, Lee J, Kim ND, Yu BP, Chung HY. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm Res. 2009; 58:143–150. PMID: 19199090.

19. Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim YH, Kim K, Paik YK. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005; 433:541–545. PMID: 15690045.

20. Goto S, Takahashi R, Araki S, Nakamoto H. Dietary restriction initiated in late adulthood can reverse age-related alterations of protein and protein metabolism. Ann N Y Acad Sci. 2002; 959:50–56. PMID: 11976185.

21. Wang C, Maddick M, Miwa S, Jurk D, Czapiewski R, Saretzki G, Langie SA, Godschalk RW, Cameron K, von Zglinicki T. Adult-onset, short-term dietary restriction reduces cell senescence in mice. Aging (Albany NY). 2010; 2:555–566. PMID: 20844316.

22. Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982; 215:1415–1418. PMID: 7063854.

23. Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009; 51:212–223. PMID: 19447517.

24. Wenfeng Z, Yakun W, Di M, Jianping G, Chuanxin W, Chun H. Kupffer cells: increasingly significant role in nonalcoholic fatty liver disease. Ann Hepatol. 2014; 13:489–495. PMID: 25152980.

25. Kanters E, Pasparakis M, Gijbels MJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJ, Clausen BE, Förster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MP. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003; 112:1176–1185. PMID: 14561702.
Fig. 1
Daumone treatment ameliorated proinflammatory cytokines in the livers of old mice. (A) Macrophage infiltration established by F4/80 immunohistochemical staining in the liver tissue. Brown, F4/80; Blue, hematoxylin. (B) F4/80 positive area was quantified by Image Pro and presented as the mean±SE of 3 mice/group. (C~G) Liver mRNA levels were determined by real-time qRT-PCR and are presented as the mean±SE of 10 mice/group. *p<0.05 vs. Y, †p<0.05 vs. O. Y, young mice; O, old mice. Magnification, 200x; scale bar, 50 µm.
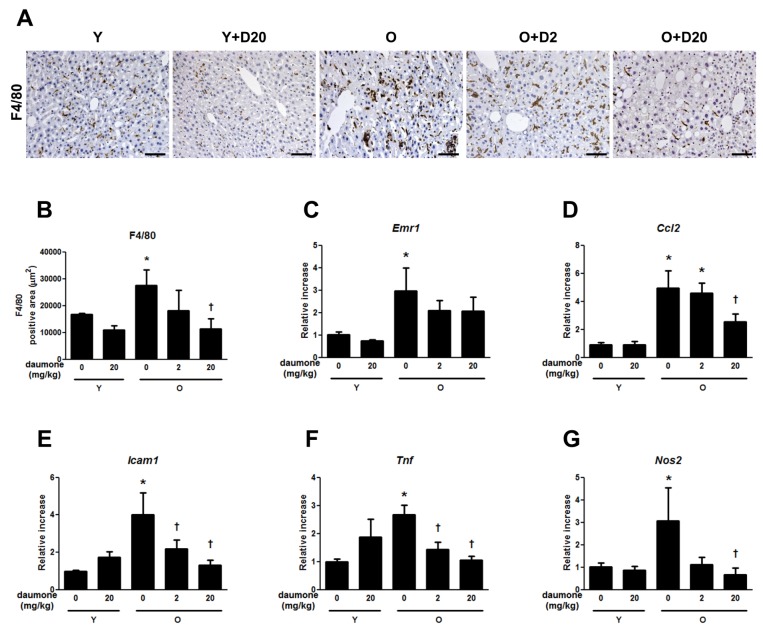
Fig. 2
Daumone attenuated NF-κB signaling in Hep G2 cells. The cells were pretreated with daumone for 6 hours before the addition of TNF-α (5 ng/ml) for 1 hour. Protein levels of (B) nuclear factor-κB (NF-κB)-p65 and (C) IκBα phosphorylation were determined by Western blot analysis. (A) Presented immunoblots. Data are presented as the mean±SE of 4 experiments. *p<0.05 vs. control, †p<0.05 vs. TNF-α only. p65, nuclear factor-κB-p65; IκB, inhibitor of nuclear factor-κB.
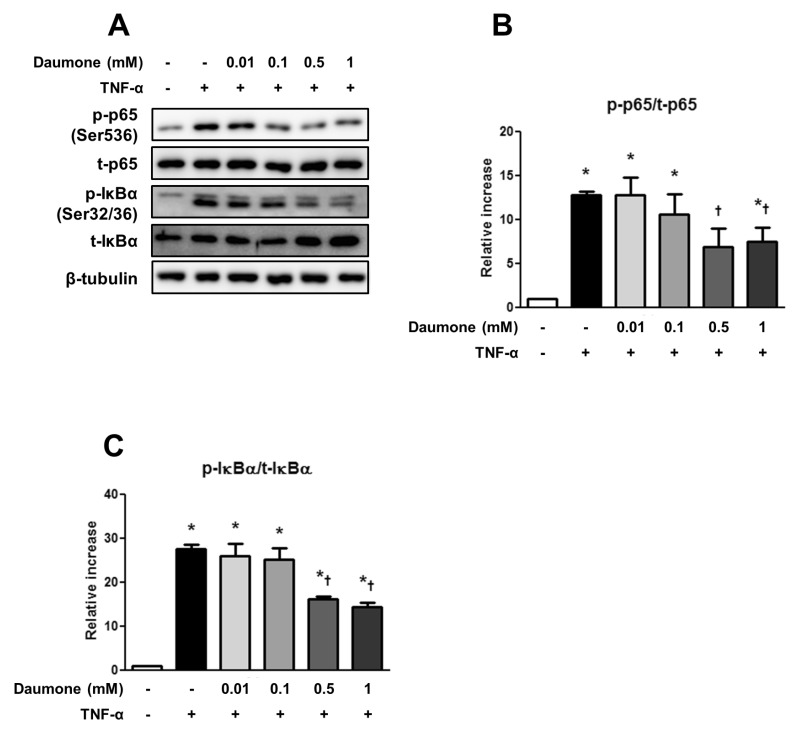
Table 2
General characteristics of experimental animals
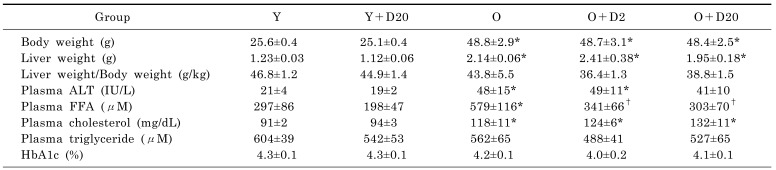
Data are presented as the mean±SE of 10 mice/group. *p<0.05 vs. Y, †p<0.05 vs. O. FFA, free fatty acids; ALT, alanine transaminase; Y, young mice; Y+D20, young mice treated with daumone (20 mg/kg); O, old mice; O+D2, old mice treated with daumone (2 mg/kg); O+D20, old mice treated with daumone (20 mg/kg).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download