Abstract
5-Lipoxygenase (5-LO) plays a pivotal role in the progression of atherosclerosis. Therefore, this study investigated the molecular mechanisms involved in 5-LO expression on monocytes induced by LPS. Stimulation of THP-1 monocytes with LPS (0~3 µg/ml) increased 5-LO promoter activity and 5-LO protein expression in a concentration-dependent manner. LPS-induced 5-LO expression was blocked by pharmacological inhibition of the Akt pathway, but not by inhibitors of MAPK pathways including the ERK, JNK, and p38 MAPK pathways. In line with these results, LPS increased the phosphorylation of Akt, suggesting a role for the Akt pathway in LPS-induced 5-LO expression. In a promoter activity assay conducted to identify transcription factors, both Sp1 and NF-κB were found to play central roles in 5-LO expression in LPS-treated monocytes. The LPS-enhanced activities of Sp1 and NF-κB were attenuated by an Akt inhibitor. Moreover, the LPS-enhanced phosphorylation of Akt was significantly attenuated in cells pretreated with an anti-TLR4 antibody. Taken together, 5-LO expression in LPS-stimulated monocytes is regulated at the transcriptional level via TLR4/Akt-mediated activations of Sp1 and NF-κB pathways in monocytes.
Monocytes play a central role in several pathophysiological conditions, when the progression of cardiovascular disease stems, from underlying inflammatory reactions [1,2]. Lipopolysaccharide (LPS) is a glycolipid component of the gram-negative bacterial cell wall and a major inflammatory cytokine that induces inflammatory responses by activating monocytes [3,4,5], and 5-lipoxygenase (5-LO) is a potent proinflammatory mediator in several inflammatory diseases, including atherosclerosis [6,7,8]. However, mechanisms responsible for the LPS-induced expression of 5-LO in monocytes remain unknown.
Several independent studies have indicated LPS in conjunction with LPS-binding protein, binds to CD14 and transmembrane Toll-like receptor 4 (TLR4) on the surfaces of a variety of cells, including monocytes [9,10]. It is also known that LPS stimulation of monocytes effects the generations of a number of inflammatory mediators, including 5-LO, and recent studies indicate that prolonged exposure to LPS upregulates FLAP expression in human monocytes [11]. The involvement of LPS in the modulation of 5-LO suggests an important interaction between bacterial infection and the development of 5-LO-mediated inflammation; furthermore, products of the 5-lipoxygenase (5-LO) pathway, which metabolizes free arachidonic acid to produce proinflammatory leukotrienes (LT) [12], have been implicated in the development and progression of atherosclerosis [13,14].
The cellular activity of 5-LO is regulated in a complex manner that involves different signaling pathways [15,16]. In particular, 5-LO expression is enhanced on monocyte cells by inflammatory stimuli via an Akt-dependent pathway [17,18], and Akt is an important mediator of signal transduction and a key player in the regulation of cellular processes. Furthermore, the activation of 5-LO in cells involves its phosphorylation by Akt. Akt has also been implicated in a variety of proinflammatory events, and its activation and phosphorylation are crucial steps in the signal transduction cascade induced by extracellular stimuli, which supports a link between the Akt pathway and 5-LO expression during the development of atherosclerosis.
In this study, 5-LO expression was found to be strongly induced by the TLR4 acvivation in monocytes. We further investigated the mechanisms by which TLR4 signaling regulates 5-LO expression in these cells and found that the Akt is the major signaling pathway that contributes to TLR4-dependent 5-LO induction. Moreover, an Akt pathway appears to increase 5-LO expression through activation of the Sp1 and NF-κB transcription factors in monocytes.
LPS from Escherichia coli was purchased from Sigma-Aldrich (Saint Louis, MO). pGL3 basic vector, pRL CMV vector, and dual luciferase reporter assay kits were purchased from Promega (Madison, WI). DNeasy Tissue Kits and QIAprep Spin Kits were supplied by Qiagen (GmhH, Germany). The various signal pathway inhibitors used were acquired from Calbiochem (Ra Jolla, CA) and Sigma (St. Louis, MO). 5-LO antibody were purchased from Santa Cruz Biotechnology (Beverly, MA). Akt, phosphospecific antibody against Akt and IKK were from Cell Signaling Technology (Beverly, MA). Purified anti-human TLR4 antibody was from eBioscience (San Diego, CA). Horseradish peroxidase (HRP)-conjugated IgG (Santa Cruz Biotechnology, Santa Cruz, MA) was used as the secondary antibody.
THP-1 cells (a human monocytic leukemia cell line) were purchased from the ATCC (Manassas, VA, USA). Cells were grown in RPMI 1640 medium (Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (FBS), antibiotic-antimycotic, and L-glutamine (Life Technologies), and maintained at 37℃ in a humidified 5% CO2/95% air atmosphere. After reaching confluence, cells were detached from T75 culture flasks by gentle scraping, washed, and resuspended in a complete medium.
Monocytes were grown to 90~95% confluence in 12-well plates. Separately, 1 µg of plasmid DNA and 2 µl of Lipofectamine LTX reagent (Invitrogen, CA) were diluted in 50 µl of Opti-MEM medium (GIBCO, NY), mixed, and incubated at room temperature for 30 min. And then the diluted mixed solution was added to the cells. Cells were then incubated in plates at 37℃ for 6 h, and after removing the conditioned medium, grown in fresh medium containing 10% FBS for 24 h, and then treated or not with LPS. Cell lysates were prepared using passive lysis buffer (Promega assay system; Promega, WI) and luciferase activities were measured according to the manufacturer's instructions for the dual luciferase reporter assay (Promega, WI). All firefly luciferase values were normalized versus Renilla luciferase to compare transfection efficiencies.
The levels of 5-LO expression, Akt and IKK phosphorylation were measured by Western blotting. Monocyte cell lysates were separated on 8% sodium dodecyl sulphate (SDS)-polyacrylamide gels, and transferred electrophoretically onto nitrocellulose membranes. Membranes were blocked with 5% skim milk in tris-buffered saline containing Tween 20 (TBST), and then incubated with anti-5-LO (1 : 1,000) in a blocking buffer. After the blots were incubated with the horseradish peroxidase (HRP)-conjugated secondary antibody (1 : 3,000), chemiluminescence intensities were measured using the LAS-3000 SYSTEM (Fuji Photo Film, Japan). Membranes were re-blotted with an anti-β-actin antibody (MP Biomedicals, Aurora, Ohio) as an internal control.
To determine the potential role for LPS in the transcriptional regulation of 5-LO expression, we initially examined the promoter activity of 5-LO in LPS-treated monocytes. As shown in Fig. 1A and 1B, the levels of 5-LO promoter activity in LPS-treated monocytes were significantly increased in a concentration- and time-dependent manners. In addition, stimulation of monocytes with various concentrations of LPS was found to increase 5-LO protein expression in a concentration-dependent manner (Fig. 1C). These results suggest that LPS increases 5-LO expression via an enhanced up-regulation of 5-LO transcription in monocytes.
To determine the role played by various signaling proteins such as MAPKs and the Akt in LPS-induced 5-LO expression, we examined their pharmacological inhibitors on LPS-induced 5-LO expression in monocytes. In this study, monocytes were pretreated with various MAPK inhibitors including PD98059 (a ERK inhibitor), SB203580 (a p38 MAPK inhibitor), and SP600125 (a JNK inhibitor), and an inhibitor for Akt pathway (AI), and then stimulated with LPS. As shown in Fig. 2A, LPS-induced 5-LO expression was significantly inhibited by an Akt inhibitor, but not by MAPK inhibitors. In addition, LPS significantly and time-dependently increased the level of phosphorylated Akt, but not total Akt, indicating a pivotal role of LPS on the activation of Akt pathway in monocytes.
To identify transcription factors involved in LPS-induced 5-LO expression, this study determined whether Sp1 and NF-κB is involved in LPS-induced 5-LO transcription in monocytes. As shown in Fig. 2C and 2D, the luciferase reporter activities of Sp1 and NF-κB in LPS-treated monocytes were significantly increased in a time-dependent manner. These results indicate that the Akt/Sp1 and Akt/NF-κB pathways mediate LPS-induced 5-LO transcription in monocytes.
To determine the links between Akt pathway and the activities of Sp1 and NF-κB, the luciferase reporter activities of these transcription factors in LPS-treated monocytes were investigated in the presence or absence of an Akt inhibitor. As shown in Fig. 3A, AI inhibited the increase in the LPS-induced 5-LO promoter activity, and also markedly attenuated the increase in the activities of Sp1 and NF-κB (Fig. 3B). Moreover, the increased phosphorylation of IKK by LPS was also attenuated by pre-treatment with AI in monocytes (Fig. 3C), suggesting an important role for Akt signaling pathway on the activation of Sp1 and NF-κB in LPS-stimulated monocytes.
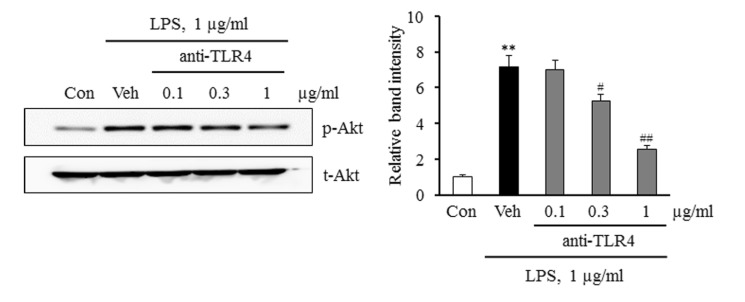
To determine whether the LPS-induced activation of Akt was mediated via the activation of TLR4, Akt phosphorylation was investigated in LPS-stimulated monocytes pretreated with a TLR4 functional blocking antibody. As shown in Fig. 4, the increase in LPS-induced Akt phosphorylation was attenuated in a concentration-dependent manner in monocytes pretreated with an anti-TLR4 antibody. These results suggest that TLR4 on the surface of monocytes is a pivotal mediator linking LPS and Akt pathway in monocytes.
In this study, we examined molecular mechanisms that regulate LPS-induced 5-LO expression in monocytes. Our results show that LPS enhances the activities of Sp1 and NF-κB in monocytes, and that these activations increase 5-LO promoter activity and protein expression. We found that increased Sp1 and NF-κB activities and the subsequent expression of 5-LO by LPS were significantly attenuated by inhibiting the Akt pathway. Furthermore, LPS-enhanced Akt phosphorylation was attenuated markedly by a TLR4 functional blocking antibody. These results support the hypothesis that LPS enhances 5-LO expression in monocytes via the TLR4-mediated up-regulation of the activities of Sp1 and NF-κB and activation of the Akt pathway.
Atherosclerosis is known to be a major contributor to cardiovascular disease [19,20]. Bacterial infection, such as lipopolysaccharide (LPS) has also been shown to be an important factor responsible for triggering atherosclerosis and the associated cardiovascular diseases [21]. LPS is a constituent of the cell wall of gram-negative bacteria and major cause of inflammatory response associated with bacterial infection. In fact, elevated serum LPS levels have been reported to increase the risk of atherosclerosis development in man [22] and in mouse models [23], and the modulatory effects of inflammatory stimuli on 5-lipoxygenase-activating protein (FLAP) gene expression in monocytes have attracted considerable interest [24]. To investigate these relationship, we evaluated the effects of LPS on the regulation of 5-LO expression in monocytes. Although our results suggest that activated monocytes play an important role in atherosclerosis, the role played by 5-LO signaling in LPS-mediated pathophysiological processes that lead to the progression of atherosclerosis has not been fully determined.
The present study provides insight of the mechanism whereby LPS modulates 5-LO gene expression in monocytes. Previous studies have shown that LPS activates MAPKs and Akt pathways in several cell types. In the present study, the induction of 5-LO protein expression by LPS was attenuated by AI (an Akt inhibitor), but not by MAPK inhibitors including PD98059 (an ERK inhibitor), SP600125 (a JNK inhibitor), or SB203580 (a p38 inhibitor), showing that the Akt signaling pathway participates in LPS-induced 5-LO expression. In addition, it has been shown 5-LO expression is regulated at the transcriptional level [25,26]. One of these studies showed that the promoter activity of LPS-stimulated p5LO-213 in monocytes was approximately 6.2 (±0.4) times higher than that in untreated controls. In our previous study [25], sequence analysis of the region between nt -213 and +1 demonstrated the presence of consensus binding sites for Sp1 and NF-κB. This result was confirmed by observations of the reporter activities of Sp1 and NF-κB, as LPS significantly enhanced their reporter activities and 5-LO promoter activity, which showed Sp1 and NF-κB are essential transcription factors for LPS-induced 5-LO transcription. Furthermore, LPS-induced reporter activities of Sp1 and NF-κB were significantly inhibited by AI, an Akt inhibitor. Accordingly, we found that LPS-induced 5-LO expression occurs via Akt-mediated Sp1 and NF-κB pathways.
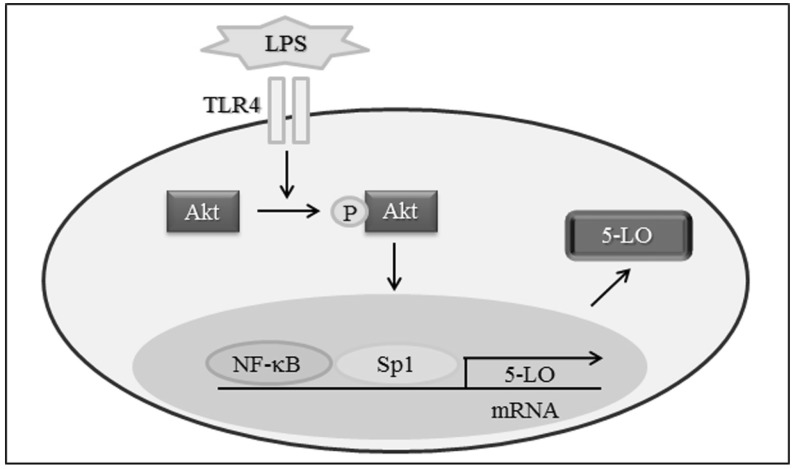
In the present study, 5-LO expression was significantly elevated in LPS-stimulated monocytes, and this elevation was associated with increased 5-LO promoter activity. The Sp1 and NF-κB transcription factors were found to be essential for LPS-induced 5-LO transcription, and increases in Sp1 and NF-κB activity induced by LPS were attenuated by inhibiting the Akt pathway. Furthermore, TLR4 functional blocking antibody attenuated Akt phosphorylation and 5-LO up-regulation in monocytes induced by LPS. Collectively, these results suggest that LPS-induced 5-LO transcription is mediated via the TLR4 mediated activations of Sp1 and NF-κB through the Akt signaling pathway (Fig. 5). This finding provides novel options for therapeutic interventions aimed at regulating 5-LO transcription in atherosclerosis.
References
1. Badimon L, Storey RF, Vilahur G. Update on lipids, inflammation and atherothrombosis. Thromb Haemost. 2011; 105(Suppl 1):S34–S42. PMID: 21479344.

2. Hansson GK. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost. 2009; 7(Suppl 1):328–331. PMID: 19630827.

3. Gupta H, Dai L, Datta G, Garber DW, Grenett H, Li Y, Mishra V, Palgunachari MN, Handattu S, Gianturco SH, Bradley WA, Anantharamaiah GM, White CR. Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide. Circ Res. 2005; 97:236–243. PMID: 16002747.

4. Kuhn AM, Tzieply N, Schmidt MV, von Knethen A, Namgaladze D, Yamamoto M, Brüne B. Antioxidant signaling via Nrf2 counteracts lipopolysaccharide-mediated inflammatory responses in foam cell macrophages. Free Radic Biol Med. 2011; 50:1382–1391. PMID: 21382476.

5. Sikorski K, Chmielewski S, Przybyl L, Heemann U, Wesoly J, Baumann M, Bluyssen HA. STAT1-mediated signal integration between IFNγ and LPS leads to increased EC and SMC activation and monocyte adhesion. Am J Physiol Cell Physiol. 2011; 300:C1337–C1344. PMID: 21346151.

6. Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res. 2010; 86:243–253. PMID: 20093252.

7. Vila L. Cyclooxygenase and 5-lipoxygenase pathways in the vessel wall: role in atherosclerosis. Med Res Rev. 2004; 24:399–424. PMID: 15170590.

8. Mehrabian M, Allayee H. 5-lipoxygenase and atherosclerosis. Curr Opin Lipidol. 2003; 14:447–457. PMID: 14501583.

9. Yonekawa K, Neidhart M, Altwegg LA, Wyss CA, Corti R, Vogl T, Grigorian M, Gay S, Lüscher TF, Maier W. Myeloid related proteins activate Toll-like receptor 4 in human acute coronary syndromes. Atherosclerosis. 2011; 218:486–492. PMID: 21782178.

10. Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008; 584:40–48. PMID: 18299127.

11. Serio KJ, Reddy KV, Bigby TD. Lipopolysaccharide induces 5-lipoxygenase-activating protein gene expression in THP-1 cells via a NF-kappaB and C/EBP-mediated mechanism. Am J Physiol Cell Physiol. 2005; 288:C1125–C1133. PMID: 15625306.
12. Zhao L, Moos MP, Gräbner R, Pédrono F, Fan J, Kaiser B, John N, Schmidt S, Spanbroek R, Lötzer K, Huang L, Cui J, Rader DJ, Evans JF, Habenicht AJ, Funk CD. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004; 10:966–973. PMID: 15322539.

13. De Caterina R, Zampolli A. From asthma to atherosclerosis--5-lipoxygenase, leukotrienes, and inflammation. N Engl J Med. 2004; 350:4–7. PMID: 14702420.
14. Jawień J. The putative role of leukotrienes in experimental atherogenesis. Pol Arch Med Wewn. 2009; 119:90–93. PMID: 19341185.

15. Rådmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. 2007; 32:332–341. PMID: 17576065.

16. Lötzer K, Funk CD, Habenicht AJ. The 5-lipoxygenase pathway in arterial wall biology and atherosclerosis. Biochim Biophys Acta. 2005; 1736:30–37. PMID: 16081317.
17. Yang HJ, Youn H, Seong KM, Yun YJ, Kim W, Kim YH, Lee JY, Kim CS, Jin YW, Youn B. Psoralidin, a dual inhibitor of COX-2 and 5-LOX, regulates ionizing radiation (IR)-induced pulmonary inflammation. Biochem Pharmacol. 2011; 82:524–534. PMID: 21669192.

18. Sánchez-Galán E, Gómez-Hernández A, Vidal C, Martín-Ventura JL, Blanco-Colio LM, Muñoz-García B, Ortega L, Egido J, Tuñón J. Leukotriene B4 enhances the activity of nuclear factorkappaB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc Res. 2009; 81:216–225. PMID: 18852255.
19. Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011; 12:204–212. PMID: 21321594.

20. Drüeke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. 2010; 6:723–735. PMID: 20978469.

21. Gitlin JM, Loftin CD. Cyclooxygenase-2 inhibition increases lipopolysaccharide-induced atherosclerosis in mice. Cardiovasc Res. 2009; 81:400–407. PMID: 18948273.

22. Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, Li PK. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008; 3:431–436. PMID: 18256376.

23. Lalla E, Lamster IB, Hofmann MA, Bucciarelli L, Jerud AP, Tucker S, Lu Y, Papapanou PN, Schmidt AM. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2003; 23:1405–1411. PMID: 12816879.

24. Serio KJ, Reddy KV, Bigby TD. Lipopolysaccharide induces 5-lipoxygenase-activating protein gene expression in THP-1 cells via a NF-kappaB and C/EBP-mediated mechanism. Am J Physiol Cell Physiol. 2005; 288:C1125–C1133. PMID: 15625306.
25. Lee SJ, Kim CE, Seo KW, Kim CD. HNE-induced 5-LO expression is regulated by NF-{kappa}B/ERK and Sp1/p38 MAPK pathways via EGF receptor in murine macrophages. Cardiovasc Res. 2010; 88:352–359. PMID: 20554538.
26. Serezani CH, Lewis C, Jancar S, Peters-Golden M. Leukotriene B4 amplifies NF-κB activation in mouse macrophages by reducing SOCS1 inhibition of MyD88 expression. J Clin Invest. 2011; 121:671–682. PMID: 21206089.

Fig. 1
Effects of LPS on 5-LO expression in monocytes. Monocytes were transiently cotransfected with the empty luciferase vector pRL CMV or 5-LO promoter constructs for 36 h, and then stimulated with the indicated concentrations of LPS for 4 h (A) or 1 µg/ml of LPS for the indicated time (B). The promoter activity of 5-LO was analyzed using a luciferase reporter assay. (C) Monocytes were stimulated with the indicated concentrations of LPS for 4 h, and the protein expression of 5-LO were analyzed by immunoblotting. Relative band intensity of 5-LO to β-actin was quantified, and the results were presented as the mean±SEM of 4-5 independent experiments performed in triplicate. *p< 0.05, **p<0.01 vs. value at concentration 0 or time 0.
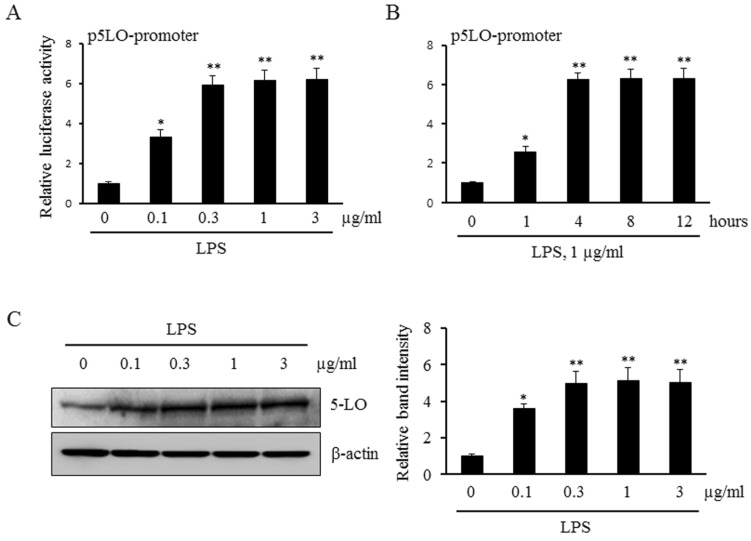
Fig. 2
Effects of various signal pathway inhibitors on LPS-induced 5-LO expression. (A) Monocytes were pre-treated with MAPK inhibitors including PD98059 (PD, 30 mM), SP600125 (SP, 30 mM), SB203580 (SB, 30 mM), or AI (3 mM) for 30 min, and then stimulated with 1 µg/ml of LPS. 5-LO expressions were analyzed by immunoblotting, and data were presented as means±SEM from 4~6 independent experiments. **p<0.01 vs. value of control (Con), ##p<0.01 vs. value of vehicle (Veh). (B) Monocytes were stimulated with 1 µg/ml of LPS for the indicated times. The cell lysates were analyzed for phosphorylated (p-Akt) and total Akt (t-Akt) by Western blotting. Relative intensity of p-Akt to t-Akt was quantified, and data were presented as means±SEM from 6~7 independent experiments. *p<0.05, **p<0.01 vs. value at time 0. (C and D) Monocytes were transiently transfected with the Sp1 and NF-κB luciferase reporter constructs for 36 h, and then stimulated with LPS for the indicated times. Sp1 and NF-κB activities were analyzed using luciferase reporter assays. Data were presented as means±SEM of 4-5 independent experiments performed in triplicate. **p<0.01 vs. value at time 0.
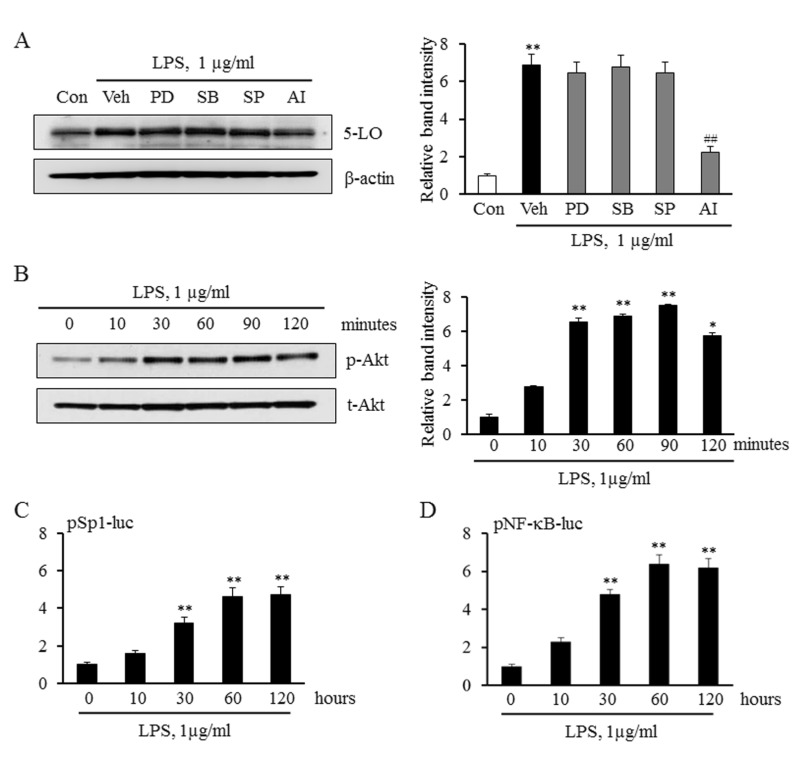
Fig. 3
Role of Akt on LPS-induced 5-LO expression mediated by Sp1 and NF-κB signaling pathways. (A) Monocytes were transiently cotransfected with the empty luciferase vector pRL CMV or 5-LO promoter constructs for 36 h, and then stimulated with LPS (1 µg/ml) in the presence of AI (3 µm). 5-LO promoter activities were determined using a luciferase reporter assay. The data is presented as the mean±SEM from 5~6 independent experiments. (B) Monocytes were transiently transfected with the Sp1 and NF-κB luciferase reporter constructs for 36 h. After pretreatment with AI (3 µm) for 30 min, cells were treated with LPS (1 µg/ml). Sp1 and NF-κB activities were analyzed using luciferase reporter assays, and data were presented as the mean±SEM from 5~6 independent experiments. **p<0.01 vs. control (Con), ##p<0.01 vs. vehicle (Veh). (C) Monocytes were stimulated with LPS (1 µg/ml) for the indicated time in the presence or absence of AI (3 µm). The cell lysates were analysed for the phosphorylated levels of IKK (pIKK). Relative intensity to β-actin was presented as the mean±SEM from 6~7 independent experiments. **p<0.01 vs. value at time 0.
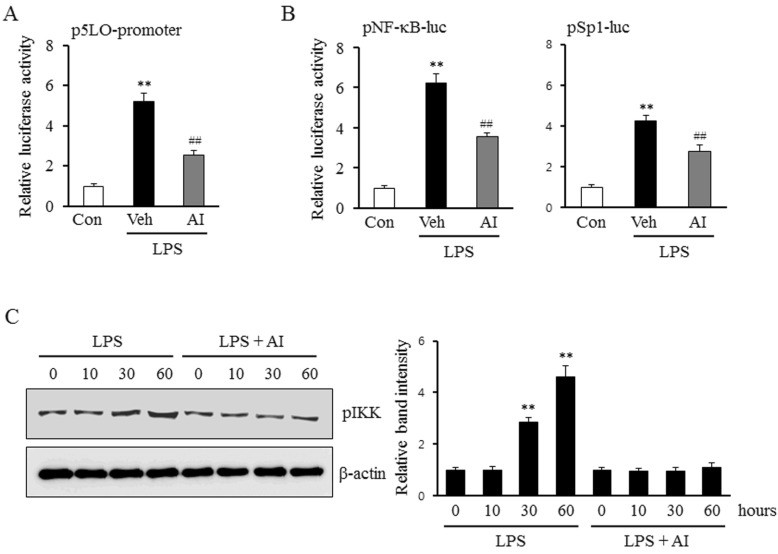
Fig. 4
Involvement of TLR4 pathway in Akt phosphorylation induced by LPS. Monocytes were pre-treated with a TLR4 functional blocking antibody (anti-TLR4) for 30 min, and then stimulated with LPS (1 µg/ml). Cell lysates were analyzed for the total (t-Akt) and phosphorylated levels of Akt (p-Akt) using Western blotting. Relative band intensity of p-Akt to t-Akt was quantified, and data were presented as the mean±SEM from 5~6 independent experiments. **p<0.01 vs. control (Con), #p<0.05, ##p<0.01 vs. vehicle (Veh).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download