Abstract
Excessive microglial activation and subsequent neuroinflammation lead to synaptic loss and dysfunction as well as neuronal cell death, which are involved in the pathogenesis and progression of several neurodegenerative diseases. Thus, the regulation of microglial activation has been evaluated as effective therapeutic strategies. Although dieckol (DEK), one of the phlorotannins isolated from marine brown alga Ecklonia cava, has been previously reported to inhibit microglial activation, the molecular mechanism is still unclear. Therefore, we investigated here molecular mechanism of DEK via extracellular signal-regulated kinase (ERK), Akt and nicotinamide adenine dinuclelotide phosphate (NADPH) oxidase-mediated pathways. In addition, the neuroprotective mechanism of DEK was investigated in microglia-mediated neurotoxicity models such as neuron-microglia co-culture and microglial conditioned media system. Our results demonstrated that treatment of anti-oxidant DEK potently suppressed phosphorylation of ERK in lipopolysaccharide (LPS, 1 µg/ml)-stimulated BV-2 microglia. In addition, DEK markedly attenuated Akt phosphorylation and increased expression of gp91phox, which is the catalytic component of NADPH oxidase complex responsible for microglial reactive oxygen species (ROS) generation. Finally, DEK significantly attenuated neuronal cell death that is induced by treatment of microglial conditioned media containing neurotoxic secretary molecules. These neuroprotective effects of DEK were also confirmed in a neuron-microglia co-culture system using enhanced green fluorescent protein (EGFP)-transfected B35 neuroblastoma cell line. Taken together, these results suggest that DEK suppresses excessive microglial activation and microglia-mediated neuronal cell death via downregulation of ERK, Akt and NADPH oxidase-mediated pathways.
Microglial cells are the resident innate-immune cells in the brain, which play a physiological role of immune surveillance and host defense. However, it is generally accepted that excessive microglial activation and subsequent neuroinflammation lead to synaptic loss and dysfunction as well as neuronal cell death. These changes are involved in the pathogenesis and progression of several neurodegenerative diseases [1,2].
Microglia, in response to brain injury or immunological stimuli, produce excess nitric oxide (NO) and proinflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), which are considered to contribute to neuronal cell death and neurodegenerative processes. Multiple signaling pathways are involved in the expression of these molecules. Protein kinases such as mitogen-activated protein kinases (MAPKs), protein kinase C (PKC) and phosphoinositide 3 kinase (PI3K)/Akt are involved, while nuclear factor κB (NF-κB) are recruited in microglial activation pathways [3,4].
In addition, the role of nicotinamide adenine dinuclelotide phosphate (NADPH) oxidase has been emphasized in the microglial activation process. The NADPH oxidase, as the major source of microglial reactive oxygen species (ROS), is an enzyme complex composed of various membrane and cytosol subunits depending on cell types. Nox1, Nox2 and Nox4 isotypes are expressed as the catalytic subunits of NADPH oxidase in microglia [5,6]. Nox2 (also known as gp91phox) is the most responsive in activated microglia [6]. Nox2-dependent NADPH oxidase consists of two membranebound subunits and four cytosolic subunits in microglia. Upon phosphorylation by specific kinases, the cytosolic subunits form a complex and translocate to the membrane to dock with the membrane subunits and activate NADPH oxidase complex. Then, the activated NADPH oxidase generates the superoxide anion (·O2-) by reducing O2, that can be converted to other ROS molecules [7,8]. A growing body of evidence has indicated that NADPH oxidase and NADPH oxidase-derived ROS are associated with MAPKs, Akt and NF-κB, and thereby play an important role in microglial activation and neuroinflammation [3,9,10,11].
Many compounds derived from plants have been widely investigated as candidates for medicinal application. It has been previously reported that dieckol (DEK) isolated from marine brown alga Ecklonia cava (EC) exhibits anti-inflammatory and antitumor activity as well as free radical scavenging activity [12,13]. In the central nervous system, DEK was shown to inhibit cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in activated microglia following LPS stimulation [14]. However, the molecular mechanism underlying neuroprotective activity of DEK still remains to be elucidated.
Therefore, we further investigated here whether DEK inhibits microglial activation via ERK, Akt, and NADPH oxidase-mediated pathways in activated microglia. In addition, we found, using a neuron-microglia co-culture system and microglial conditioned media system, that DEK inhibits neuronal cell death following excess activation of adjacent microglial cells.
Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin and Alexa Fluor 488-conjugated goat anti-rabbit antibodies were obtained from Invitrogen (Carlsbad, CA, USA). LY294002, Wortmannin and diphenyleneiodium (DPI) were purchased from Calbiochem (La Jolla, CA, USA). Antibodies against extracellular signal-regulated kinase (ERK), phospho-ERK, Akt, phospho-Akt, nuclear factor κB p65 (NF-κB p65) and inhibitor of κBα (IκBα) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against p38, phospho-p38 and inducible nitric oxide synthase (iNOS) were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibody against TATA binding protein (TBP) was purchased from Abcam (Cambridge, UK). Antibody against gp91phox was purchased from BD biosciences (San Jose, CA, USA). Horseradish peroxide (HRP)-conjugated immunoglobulin G (IgG) antibody was purchased from Vector Laboratories (Burlingame, MA, USA). All the other reagents were purchased from Sigma (St. Louis, MO, USA), unless indicated.
DEK was kindly supplied by BotaMedi, Inc. (Seoul, Republic of Korea). Briefly, the whole plant of marine brown alga Ecklonia cava was collected from the Jeju Island coast in the Republic of Korea. The dried Ecklonia cava powder was extracted three times with 70% aqueous ethanol (EtOH) and then filtered. The filtrate was evaporated at 50℃ to isolate the ethanol extract. After the EtOH extract had been suspended in distilled water, it was partitioned two times with n-butanol. The n-butanol fraction was evaporated in a vacuum, and was subjected to ODS column chromatography. The DEK compound was finally purified by LH-20 column chromatography and the purified DEK was then confirmed by comparing their mass spectrometry, 1H-nuclear magnetic resonance (NMR) and 13C-NMR data to those in the reported literature [15].
Primary microglial cells were prepared from cerebral cortices of 1-day-old Sprague-Dawley rats as previously described [16]. BV-2 microglia cells [17] were a generous gift from Dr. E. Joe (Ajou University, Republic of Korea). HT-22 neurons, an immortalized hippocampal neuronal cell line [18,19], were obtained from Dr. B.H. Lee (Gachon University of Medicine and Science, South Korea). Enhanced green fluorescent protein (EGFP)-transfected B35 rat neuroblasoma cells (ATCC, CRL-2754) [20] were used to establish a neuron and microglia co-culture system. BV-2 microglia, HT-22 neurons and B35 rat neuroblastoma cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin, and maintained at 37℃ in a humidified atmosphere of 5% CO2.
Neurotoxicity analysis of microglial conditioned media has been well established in previous reports [21,22,23]. BV-2 microglial cells were pretreated with DEK for 6 h, then the culture supernatants were discarded to eliminate any direct effect of DEK on HT-22 neurons. The cells were washed and further incubated with lipopolysaccharide (LPS, 1 µg/ml) for 24 h in the absence of DEK. The used culture media were collected from the culture dishes and centrifuged to remove the detached cells. Then, the supernatants were used as microglial conditioned media. HT-22 neurons were treated with various groups of microglial conditioned media for 24 h and neuronal viabilities were assessed by MTT assay and phase contrast microscopy.
EGFP-transfected B35 rat neuroblastoma cells (B35-EGFP) were added to BV-2 microglia-plated wells and co-cultured (2.5 : 1 ratio) at the density of 1.5×105 cells/well in 48-well plate, as previously described [3]. An aminoglycoside antibiotic G418 (1 mg/ml) was used to select EGFP-transfected cells containing a neomycin-resistant gene. BV-2 microglial cells were treated with DEK for 6 h and then, washed before adding B35-EGFP cells to eliminate direct effect of DEK on B35-EGFP neurons. In the absence of DEK, neuron-microglia co-culture system was stimulated by LPS for 24 h. The numbers of B35-EGFP neuronal cells were assessed by counting EGFP-positive cells under a fluorescent microscope (Olympus IX70, Tokyo, Japan). Images of five random fields per well were captured and counted.
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] was used to examine the effect of DEK on cell viability, as previously described [2]. After treatment, 250 µl MTT (2 mg/ml) was added to each well and the mixture further incubated for 2 h. The liquid in each well was then aspirated and 500 µl dimethyl sulfoxide (DMSO) was added, mixed thoroughly on a shaker for 30 min. Absorbance was subsequently read at 540 nm using a microplate reader (Model 550, Bio-Rad, Hercules, CA, USA).
LPS-stimulated nitric oxide (NO) production was measured using the Griess reagent as previously reported [2]. Microglial cells were pretreated with DEK for 1 h and then stimulated with LPS (1 µg/ml) in the presence or absence of DEK for 24 h. The supernatants were mixed with equal amounts of Griess reagent. Samples were incubated at room temperature for 10 min and absorbance was subsequently read at 540 nm using a microplate reader (Model 550, Bio-Rad, Hercules, CA, USA).
The amount of pro-inflammatory cytokines released into the culture medium was measured using mouse IL-6 and TNF-α ELISA kits based on the quantitative sandwich enzyme immunosorbent technique. The assay was performed according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA) and the absorbance was read at a wavelength of 450 nm using a microplate reader (Model 550, Bio-Rad, Hercules, CA, USA).
DPPH (1,1-diphenyl-2-picrylhydrazyl) is a stable free radical that has a deep violet color in solution, and it shows a strong absorption at 517 nm due to its odd electron. Upon reduction by an antioxidant, the absorption disappears and the resulting decolorization (yellow). Ten µl of DEK was added to 190 µl of DPPH (0.15 mM) and mixed vigorously. The mixture was incubated in the dark at room temperature for 2 h, and the absorbance was read at 517 nm using a microplate reader (Tecan, Sunrise, AT, USA).
DCF-DA (2',7'-dichlorofluo-rescein diacetate) is a non-fluorescent acetylated form which diffuses into cell. The esterases cleave the acetate groups on DCF-DA within the cells, and the resulting reduced probe DCFH is oxidized by ROS, yielding the fluorescent product polar 2',7'-dichlorofluorescein (DCF). BV-2 microglial cells were seeded in a 96-well tissue culture plate at a density of 2×104 cells/well containing 200 µl medium. After stabilization for 12 h, the cells were pretreated with DEK for 1 h and then stimulated with LPS (1 µg/ml) in the presence or absence of DEK for an additional 30 min at 37℃. Then, the cells were incubated in DCF-DA (50 µM, 30 min) and fluorometric analysis was performed with the excitation/emission wavelength (485 nm/535 nm) using a Perkin Elmer LS-5B spectrofluorometer (Becton Dickinson, Mountain View, CA, USA).
Cells were harvested from the culture dishes with trypsin and centrifuged at 12,000 ×g for 5 min at 4℃. Cytoplasmic and nuclear protein preparations were performed as previously described [2]. Briefly, cell pellets were resuspended in ice-cold hypotonic buffer [10 mM Hepes (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT), 10 µg/ml aprotinin, 0.2 mM phenylmethylsulfonyl fluoride (PMSF)]. After 10 min, the cytoplasmic extract was obtained as supernatant by centrifuging for 5 min. Nuclear pellets were obtained using ice-cold high-salt buffer [20 mM Hepes (pH 7.9), 20% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, and 0.2 mM PMSF].
Western blot analysis was performed as described [2]. The membranes were probed with the following primary antibodies: ERK and phospho-ERK (1 : 1000), p38 and phospho-p38 (1 : 1000), Akt and phospho-Akt (1 : 1000), gp91phox (1 : 1000), iNOS (1 : 1000), NF-κB p65 (1 : 1000), IκBα (1 : 1000), TBP (1 : 1000) and β-actin (1 : 5000).
Dose of DEK treatment was determined based on cytotoxicity data and dose-dependency data (Fig. 1). BV-2 microglial cells were treated for 25 h with different concentrations of DEK. The cytotoxic dose of DEK was examined in BV-2 microglial cells using MTT assay (Fig. 1A). DEK did not show any toxicity at concentrations less than 100 µM. Therefore, experiments were accomplished at a concentration of 50 µM of DEK on the basis of cytotoxicty data (Fig. 1A) and dose-dependency data in BV-2 microglial cell line and primary microglia, respectively shown in Fig. 1B and 1C.
To assess the effect of DEK to inhibit NO release in LPS-stimulated BV-2 cell line and primary microglia, we measured the amount of NO in the culture medium using the Griess reagent. DEK pretreatment was carried out for 1 h prior to LPS stimulation. Then, microglial cells were treated with LPS (1 µg/ml) in the presence of DEK for 24 h. LPS treatment evoked robust NO release both in primary microglia and BV-2 cell line, and it was effectively suppressed by DEK (10~50 µM) treatment in a dose-dependent manner by 21.1±0.7% (10 µM) and 58.1±0.7% (50 µM) in BV-2 microglial cell line (Fig. 1B) and by 38.8±0.5% (10 µM), 54.2±0.7% (25 µM) and 71.3±0.3% (50 µM) in primary microglia (Fig. 1C), respectively.
It has been previously reported that the release of NO, IL-1β, and TNF-α is significantly reduced by DEK treatment in LPS-stimulated microglial cells [14]. We also observed these inhibitory effects of DEK in primary microglia and BV-2 cell line (Fig. 1B and C for NO, data not shown for IL-6 and TNF-α). Therefore, we investigated whether DEK protect neurons against microglia-mediated neuronal cell death both in microglial conditioned media system (Fig. 2A) and in neuron-microglia co-culture system (Fig. 2B). These two experimental models have been well established to determine microglia-mediated neurotoxicity in current days [3,21,22,23].
BV-2 microglial cells were pretreated with DEK (50 µM) for 6 h. Then, the culture supernatants were discarded to eliminate direct effect of DEK on HT-22 neurons. The microglial cells were further incubated with LPS (1 µg/ml) for 24 h in the absence of DEK. The conditioned culture media from BV-2 microglia were collected. Then, HT-22 neurons were treated with various groups of microglial conditioned media for 24 h and neuronal viabilities were assessed by MTT assay and phase contrast microscopy. Briefly, four microglial conditioned media groups were set up: (1) the conditioned media from control BV-2 cells (Control-CM); (2) the conditioned media from LPS-treated BV-2 cells (LPS-CM); (3) the conditioned media from BV-2 cells stimulated with LPS after DEK pretreatment (LPS/DEK-CM); and (4) LPS was added to the conditioned media from control BV-2 cells (Control-CM+LPS).
We found that treatment of LPS-CM markedly decreased cell viability of HT-22 neurons to 71.0±0.3% (Fig. 2A), compared with control conditioned media. Neuronal processes of most HT-22 cells treated with LPS-CM appeared to be shrunken, compared with control CM (data not shown). Cell viabilities of HT-22 neurons treated with LPS/DEK-CM were markedly increased, compared with that of LPS-CM-stimulated HT-22 neurons. These results suggest that the neurotoxic effects of LPS-CM were almost blocked by DEK pretreatment. On the other hands, addition of LPS into control CM (Control-CM+LPS) did not affect cell viabilities of resting neurons. Thus, it is indicated that HT-22 neurons are not be directly damaged by LPS.
BV-2 microglial cells were pretreated with DEK (50 µM) for 6 h and then washed before adding B35-EGFP cells to eliminate direct effect of DEK on B35-EGFP neurons. Thereafter, B35-EGFP neurons were added to BV-2 microglial cells-plated wells. In the absence of DEK, neuron-microglia co-culture system was stimulated by LPS for 24 h. The numbers of B35-EGFP neuronal cells were assessed by counting EGFP-positive cells under a fluorescent microscope. These EGFP-transfected B35-EGFP neuroblastoma cells are useful to distinguish neurons from microglia in neuron-microglia co-culture due to their fluorescence.
The cell numbers of fluorescent B35-EGFP neurons were significantly decreased to 58.83±1.8% in this neuron-microglia co-culture system after LPS stimulation, compared to unstimulated control co-cultures. These results suggest that B35-EGFP neurons might be damaged by secretory molecules from adjacent BV-2 microglia while BV-2 cells in neuron-microglia co-culture system were stimulated by LPS. DEK-pretreated microglia did not affect neuronal cell viability without LPS stimulation. The cell viabilities were not affected by LPS alone in B35-EGFP neurons (data not shown). DEK pretreatment of microglia markedly blocked the cell death of B35-EGFP neuroblastoma cells to 90.0±4.6% after LPS stimulation in the neuron-microglia co-culture system (Fig. 2B), suggesting that DEK pretreatment has a beneficial effect for the cell viability in B35-EGFP neurons against LPS stimulation in the co-culture system. DEK pretreatment of microglia itself does not affect the cell viability in B35-EGFP neurons without LPS stimulation. These results, based on both microglial conditioned media system and the neuron-microglia co-culture system, indicate that DEK might have a therapeutic potential via attenuating microglia-mediated neurotoxicity implicated in some of neurodegenerative and neuroinflammatory diseases.
The subsequent experiments were performed to investigate the modulatory effects of DEK on the signaling pathways of microglia activation. We examined the effect of DEK on the LPS-induced phosphorylation of MAPKs such as ERK and p38 kinases in BV-2 cells using Western blot analysis. Following DEK pretreatment for 1 h, BV-2 microglial cells were stimulated with LPS (1 µg/ml) in the presence of DEK (50 µM) for 30 min. DEK significantly inhibited the phosphorylation of ERK-1/2 (Fig. 3A) and p38 (Fig. 3B) by 83.4±3.1% and 82.1±0.1% respectively. DEK did not affect LPS-induced phosphorylation of c-Jun N-terminal kinase (JNK) (data not shown). Our data are different from previous report that ERK phosphorylation was not affected by DEK treatment in LPS-stimulated microglia [14]. These discrepancies might be attributed to differences of experimental methods including dose and duration of DEK treatment.
Akt phosphorylation is known to be involved in the degradation of IκB through IκB kinase (IKK) phosphorylation, and Akt/IKK/NF-κB signaling pathways are critical for LPS-stimulated microglial activation [9]. Although DEK was shown to inhibit NF-κB activation in LPS-stimulated microglia [14], Akt involvement has not been investigated yet. Thus, we investigated the effect of DEK on LPS-induced Akt-mediated pathways using Western blot analysis. After DEK pretreatment for 1 h, BV-2 microglial cells were stimulated with LPS (1 µg/ml) in the presence of DEK (50 µM) for 30 min. As shown in Fig. 4A, phosphorylation of Akt was markedly increased 30 min after LPS stimulation in BV-2 microglia, and DEK treatment significantly inhibited Akt phosphorylation by 73.31±0.5% (Fig. 4A). In addition, DEK treatment markedly attenuated nuclear translocation of NF-κB p65 by 64.7±1.1% (Fig. 4B) and degradation of IκB by 66.0±9.3% (Fig. 4C), similar to the previous report [14]. Taken together, we have shown that DEK effectively inhibits LPS-induced Akt phosphorylation and NF-κ B activation, suggesting that DEK may suppress microglial activation via Akt/IKK/NF-κB signal cascades.
NADPH oxidase is the major source of ROS production in activated microglia. NADPH oxidase-derived ROS has been evaluated as a crucial component in the microglial activation processes. Nox1, Nox2 and Nox4 isotypes are expressed as the catalytic subunits of NADPH oxidase in microglia [5,6]. Nox2 (also known as gp91phox) is the most responsive in activated microglia [6]. It has been well established that expression of the gp91phox subunit was up-regulated in activated microglia [11,24,25].
We examined here the time course of LPS (1 µg/ml)-induced expression of the gp91phox component (Fig. 5A) using Western blot analysis. The increases in gp91phox expression were significantly shown 6 h after LPS stimulation. The increased expression was maintained through 24 h. The inhibitory effect of DEK on gp91phox expression was investigated after 6 h, based on the time course shown in Fig. 5A. The results indicated that DEK (50 µM) significantly blocked the LPS-induced increase of gp91phox expression in activated microglia (Fig. 5B). In addition, we applied apocynin and diphenylene iodonium (DPI), well-known NADPH oxidase inhibitors, to investigate whether NADPH oxidase regulates the secretion of pro-inflammatory mediator NO, which is a crucial event of microglial activation, in LPS (1 µg/ml, 24 h)-stimulated BV-2 microglia. As shown in Fig. 5C, apocynin and DPI dose-dependently inhibited NO production, indicating that NADPH oxidase is crucial in microglial activation pathway.
In addition, we observed that DEK exerts ROS scavenging activity (Fig. 5D), which is consistent with the previous report [12,14]. The time course of LPS-induced intracellular ROS levels indicates that the level of the intracellular ROS increased within 5 min, and maximized at 30 min and sustained until 24 h in LPS (1 µg/ml)-stimulated BV-2 microglia (Fig. 5E). Based on these time course data, the effects of DEK on LPS-induced ROS levels were elucidated 30 min after LPS treatment. After DEK pretreatment for 1 h, BV-2 microglial cells were stimulated with LPS (1 µg/ml) in the presence of DEK (1, 10, and 50 µM) for 30 min. We found that DEK significantly reduced intracellular ROS levels in dose-dependent manner in LPS-stimulated microglia (Fig. 5F).
Taken together, Fig. 5 indicated that DEK prevented LPS-induced increase of both intracellular ROS levels and NADPH oxidase component gp91phox expression levels. The results suggest that DEK suppresses NADPH oxidase-ROS pathway through both scavenging intracellular ROS and suppressing gp91phox expression.
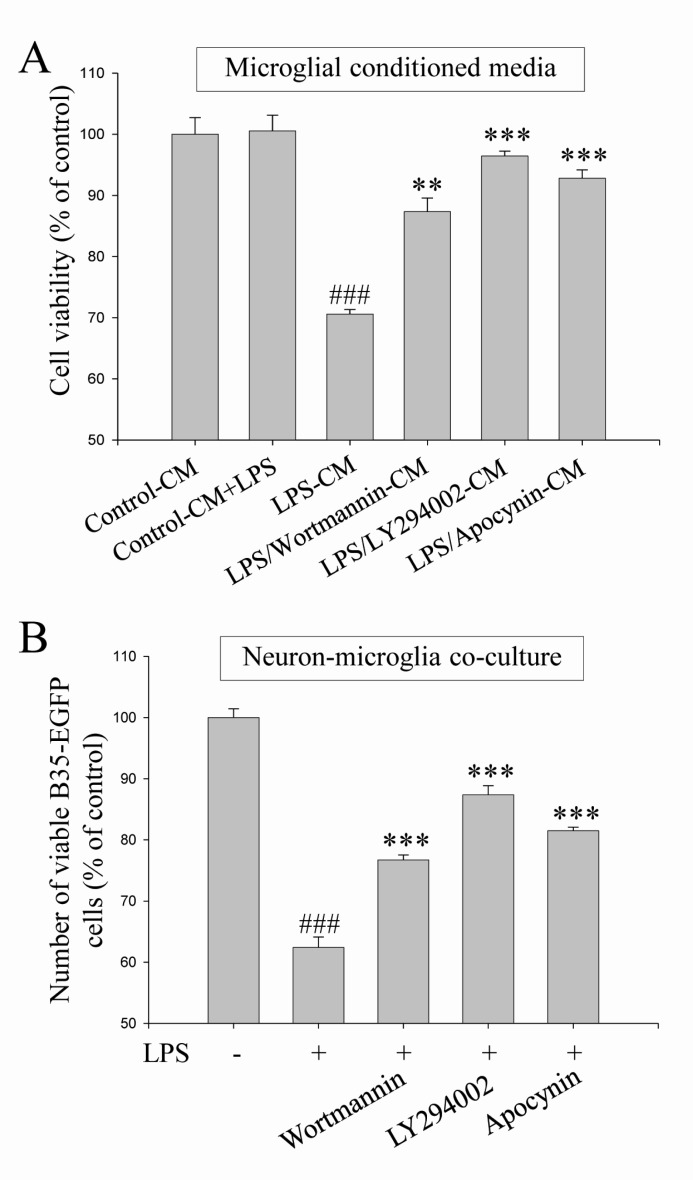
We demonstrated here that PI3K/Akt and NADPH oxidase-mediated pathways were suppressed by DEK treatment in activated microglia (Fig. 4 and 5). Therefore, neuroprotective effects of the blockade of PI3K/Akt and NADPH oxidase were investigated against microglia-mediated neuronal cell death in microglial conditioned media system and in neuron-microglia co-culture system. These two experimental models were also used to investigate the effect of DEK on microglia-mediated neuronal cell death (Fig. 2). Wortmannin (100 nM) and LY294002 (25 µM) as PI3K inhibitors and apocynin (500 µM) as a NADPH oxidase inhibitor were pretreated in both experimental systems and then, neuronal cell death was evaluated as shown in Fig. 2.
The results demonstrated that pretreatment of wortmannin, LY294002 and apocynin significantly attenuated microglia-mediated neuronal cell death in the microglial conditioned media system (Fig. 6A) and in the neuron-microglia co-culture system (Fig. 6B). Taken together, these results indicate that PI3K/Akt and NADPH oxidase-mediated signal pathways, which were suppressed by DEK, play a critical role in microglial inflammatory signaling and microglia-mediated neuronal cell death.
In the present study, we demonstrated, using both a microglia and neuron co-culture system and microglial conditioned media system, that DEK suppresses excessive microglial activation and subsequent neuronal cell death via downregulation of ERK, Akt, and NADPH oxidase-mediated pathways. These experimental systems have been successfully established in determining the neuroprotective activity of chemical compounds [3,21,22,23]. Since neurons were never exposed to DEK in this co-culture system and microglial conditioned media system, the neuroprotective effects of DEK are purely due to the suppression of microglia activation. Although this co-culture system is not the same as in vivo condition, it is believed to mimic the brain tissue where neurons and microglia mutually interact.
LPS is known to be a specific ligand for Toll-like receptor 4 (TLR4). Therefore, the LPS stimulation model is considered as a powerful experimental tool for TLR4-mediated microglial activation, which has been implicated in brain injury and neurodegeneration. It has been established that multiple signaling pathways are activated in microglial cells upon recognition of LPS by TLR4 [3]. DEK was previously shown to inhibit COX-2 and iNOS via p38 kinase and NF-κB, not ERK in activated microglia following LPS stimulation [14]. In spite of this report, the molecular mechanism underlying neuroprotective activity of DEK still remains to be elucidated. Therefore, we demonstrated here using a neuron-microglia co-culture system and microglial conditioned media system that DEK suppresses microglia-mediated neuronal cell death via suppression of microglial activation, which is mediated by downregulation of ERK, Akt, and gp91phox-mediated pathways.
A growing body of evidence has indicated that phosphorylation of MAPKs, Akt and IκB kinase (IKK) and NF-κB translocation were significantly suppressed by downregulation of intracellular ROS using ROS scavenger N-acetyl cysteine (NAC), suggesting these molecules are ROS-dependent in activated microglia [3,9,10,11,26,27]. The upstream and downstream relationships between these molecules have been proposed in several types of cells where NF-κB activity is associated with MAPKs or PI3K/Akt pathways. IκB degradation is known to be mediated through phosphorylation of IKK by Akt in activated microglia [28,29]. It is currently unclear whether MAPKs acts upstream of NF-κB in activated microglial cells, although NF-B activation via MAPKs pathway was demonstrated in other cell types [27,30].
In addition to these above pathways, we investigated the effect of DEK on NADPH oxidase-mediated pathway. We found here that blockade of NADPH oxidase using the well-known inhibitors DPI and apocynin significantly suppressed microglial pro-inflammatory mediator NO production (Fig. 5C), indicating that NADPH oxidase and NADPH oxidase-derived ROS are critical in the microglial activation pathway. We found that intracellular ROS generation was rapidly evoked within 5 min in LPS-stimulated microglia. Maximum induction was observed after 30 min and sustained until 24 h after LPS stimulation (Fig. 5E). LPS-induced intracellular ROS levels were significantly suppressed by DEK treatment in a dose-dependent manner (Fig. 5F), as consistent with the previous report [13].
On the other hand, the increases in gp91phox expression were significantly shown 6 h after LPS stimulation. The increased expression was maintained through 24 h (Fig. 5A). This gp91phox upregulation is likely to contribute to high levels of sustained intracellular ROS. Significant suppression of gp91phox expression could contribute to reduction of intracellular ROS levels in DEK-treated microglia, since gp91phox expression is the catalytic subunit of NADPH oxidase complex, the major source of microglial ROS production. Taken together, these results suggest that DEK suppresses NADPH oxidase-ROS pathway by not only scavenging intracellular ROS (Fig. 5D and F), but also suppressing gp91phox expression (Fig. 5B).
It is not clear how DEK suppresses gp91phox expression in LPS-stimulated microglia. However, it could be postulated that DEK might suppress gp91phox expression via down regulating the NF-κB pathway. The existence of typical NF-κB binding elements was demonstrated in the promoter of Nox1 and Nox4, which are also isoforms of catalytic subunits of NADPH oxidase complex. Transcriptional regulation of Nox1 and Nox4 by NF-κB was proposed in human aortic smooth muscle cells [31]. However, to our knowledge, transcriptional regulation of Nox2 by NF-κB has not been clearly elucidated yet.
The NADPH oxidase generates superoxide anion (·O2-) by reducing O2 in the extracellular space, which can be converted to other ROS molecules such as H2O2 and highly reactive hydroxyl radical (·OH) [32]. In addition, ·O2- toxicity is exacerbated by reacting with ·NO to form peroxinitrite (ONOO-), which modifies tyrosine in proteins to nitrotyrosines [33,34]. H2O2 is a diffusible molecule that can penetrate the lipid bilayer to enter the cytosol [32], where it can function as a second messenger for redox signaling [35]. It has been demonstrated that intracellular H2O2 inactivates protein tyrosine phosphatases (PTPs) by oxidizing the catalytic domain containing reactive cysteine residue, which leads to promote tyrosine phosphorylation of certain protein kinases in various cell types [35]. MAPKs phosphorylation, which is important in microglial activation signaling pathways, can be modulated by MAPKs phosphatases-1 (MKP-1) and apoptosis signal-regulating kinase-1 (ASK-1), which are known to be redox-regulated phosphatases and kinases respectively [36,37,38].
It is of interest that p38 activation was required for the phosphorylation of a cytosol subunit of NADPH oxidase complex p47phox and the subsequent ROS production in Mycobacterium tuberculosis-stimulated microglial cells [39]. In addition, NADPH oxidase-derived ROS was upstream signal molecules of p38, suggesting that neuroinflammation signals might be amplified through such mutual activation between MAPKs and NADPH oxidase-ROS pathway [38]. Similarly, there might be other positive feedback loop of mutual activation between NF-κB and NADPH oxidase-ROS pathway, considering that NF-κB regulates NADPH oxidase subunit expression via Nox1 or Nox4 transcriptional regulation [31] and, inversely, NADPH oxidase activates NF-κB through the subsequent ROS [3,9,11]. However, the existence of these possible multiple positive feedback loops in microglial activation process remains to be elucidated clearly.
Emerging evidence from several studies indicates that microglial TLR4 contributes to spinal nerve injury-induced neuropathic pain [40] and delayed neuronal cell death in a cerebral ischemia animal model [41]. These reports suggest that TLR4 signaling may play a pivotal role in the development of various neurodegenerative diseases. Previous reports have suggested that microglial TLRs may bind to not only exogenous LPS ligands but also various endogenous ligands released from damaged neurons or accumulated in certain pathological conditions [42,43]. Furthermore, we speculate that there might be crucial cross-talk in the downstream signaling pathways between TLR4 and certain microglial receptors for specific endogenous molecules such as ATP and glutamate [16], though these possibilities remain to be clearly elucidated.
In conclusion, we demonstrate evidences indicating that DEK suppresses LPS-induced excessive microglial activation and the subsequent neuronal cell death via downregulation of ERK, Akt, and NADPH oxidase-mediated pathways. It is postulated that the ROS scavenging activity of DEK seems to play a central role in the neuroprotective mechanism against microglia-mediated neurotoxicity, since these signaling pathways are known to be ROS-dependent [3,9,10,11,26,27]. Taken together, these results suggest that DEK might have a neuroprotective potential via attenuating microglia-mediated neurotoxicity implicated in the pathogenesis of neuroinflammation and neurodegeneration.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NRF-2013R1A1A2013585).
References
1. Aschner M, Allen JW, Kimelberg HK, LoPachin RM, Streit WJ. Glial cells in neurotoxicity development. Annu Rev Pharmacol Toxicol. 1999; 39:151–173. PMID: 10331080.

2. Cui Y, Wu J, Jung SC, Park DB, Maeng YH, Hong JY, Kim SJ, Lee SR, Kim SJ, Kim SJ, Eun SY. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol Pharm Bull. 2010; 33:1814–1821. PMID: 21048305.

3. Ock J, Han HS, Hong SH, Lee SY, Han YM, Kwon BM, Suk K. Obovatol attenuates microglia-mediated neuroinflammation by modulating redox regulation. Br J Pharmacol. 2010; 159:1646–1662. PMID: 20397299.

4. Lee JW, Cheong IY, Kim HS, Lee JJ, Lee YS, Kwon YS, Kim MJ, Lee HJ, Kim SS, Chun W. Anti-inflammatory activity of 1-docosanoyl cafferate isolated from rhus verniciflua in LPSstimulated BV2 microglial cells. Korean J Physiol Pharmacol. 2011; 15:9–15. PMID: 21461235.
5. Chéret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008; 28:12039–12051. PMID: 19005069.
6. Cooney SJ, Bermudez-Sabogal SL, Byrnes KR. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J Neuroinflammation. 2013; 10:155. PMID: 24344836.

8. DeLeo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J Leukoc Biol. 1996; 60:677–691. PMID: 8975869.

9. Oh YT, Lee JY, Lee J, Kim H, Yoon KS, Choe W, Kang I. Oleic acid reduces lipopolysaccharide-induced expression of iNOS and COX-2 in BV2 murine microglial cells: possible involvement of reactive oxygen species, p38 MAPK, and IKK/NF-kappaB signaling pathways. Neurosci Lett. 2009; 464:93–97. PMID: 19699266.
10. Sun HN, Kim SU, Lee MS, Kim SK, Kim JM, Yim M, Yu DY, Lee DS. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent activation of phosphoinositide 3-kinase and p38 mitogen-activated protein kinase signal pathways is required for lipopolysaccharide-induced microglial phagocytosis. Biol Pharm Bull. 2008; 31:1711–1715. PMID: 18758064.

11. Zhang L, Wu C, Zhao S, Yuan D, Lian G, Wang X, Wang L, Yang J. Demethoxycurcumin, a natural derivative of curcumin attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-kappaB signaling pathways in N9 microglia induced by lipopolysaccharide. Int Immunopharmacol. 2010; 10:331–338. PMID: 20018257.
12. Kim AR, Shin TS, Lee MS, Park JY, Park KE, Yoon NY, Kim JS, Choi JS, Jang BC, Byun DS, Park NK, Kim HR. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J Agric Food Chem. 2009; 57:3483–3489. PMID: 19338274.

13. Ryu B, Li Y, Qian ZJ, Kim MM, Kim SK. Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling: implication for chronic articular disease. Chem Biol Interact. 2009; 179:192–201. PMID: 19330880.

14. Jung WK, Heo SJ, Jeon YJ, Lee CM, Park YM, Byun HG, Choi YH, Park SG, Choi IW. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J Agric Food Chem. 2009; 57:4439–4446. PMID: 19408937.

15. Li Y, Qian ZJ, Ryu B, Lee SH, Kim MM, Kim SK. Chemical components and its antioxidant properties in vitro: an edible marine brown alga, Ecklonia cava. Bioorg Med Chem. 2009; 17:1963–1973. PMID: 19201199.

16. Eun SY, Hong YH, Kim EH, Jeon H, Suh YH, Lee JE, Jo C, Jo SA, Kim J. Glutamate receptor-mediated regulation of c-fos expression in cultured microglia. Biochem Biophys Res Commun. 2004; 325:320–327. PMID: 15522236.

17. Bocchini V, Mazzolla R, Barluzzi R, Blasi E, Sick P, Kettenmann H. An immortalized cell line expresses properties of activated microglial cells. J Neurosci Res. 1992; 31:616–621. PMID: 1578513.

18. Breyer A, Elstner M, Gillessen T, Weiser D, Elstner E. Glutamate-induced cell death in neuronal HT22 cells is attenuated by extracts from St. John's wort (Hypericum perforatum L.). Phytomedicine. 2007; 14:250–255. PMID: 17346956.

19. Maher P, Davis JB. The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci. 1996; 16:6394–6401. PMID: 8815918.

20. Schubert D, Heinemann S, Carlisle W, Tarikas H, Kimes B, Patrick J, Steinbach JH, Culp W, Brandt BL. Clonal cell lines from the rat central nervous system. Nature. 1974; 249:224–227. PMID: 4151463.

21. Cui Y, Wu J, Jung SC, Kim GO, Kyeong Ko R, Lee HJ, Yoo ES, Kang HK, Suk K, Eun SY. Neuroprotective effect of methyl lucidone against microglia-mediated neurotoxicity. Eur J Pharmacol. 2012; 690:4–12. PMID: 22683871.

22. Park GH, Jeon SJ, Ko HM, Ryu JR, Lee JM, Kim HY, Han SH, Kang YS, Park SH, Shin CY, Ko KH. Activation of microglial cells via protease-activated receptor 2 mediates neuronal cell death in cultured rat primary neuron. Nitric Oxide. 2010; 22:18–29. PMID: 19887113.

23. Wang S, Wang H, Guo H, Kang L, Gao X, Hu L. Neuroprotection of Scutellarin is mediated by inhibition of microglial inflammatory activation. Neuroscience. 2011; 185:150–160. PMID: 21524691.

24. Dohi K, Ohtaki H, Nakamachi T, Yofu S, Satoh K, Miyamoto K, Song D, Tsunawaki S, Shioda S, Aruga T. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflammation. 2010; 7:41. PMID: 20659322.

25. Zhao S, Zhang L, Lian G, Wang X, Zhang H, Yao X, Yang J, Wu C. Sildenafil attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-κB signaling pathways in N9 microglia. Int Immunopharmacol. 2011; 11:468–474. PMID: 21220057.

26. Tsai HH, Lee WR, Wang PH, Cheng KT, Chen YC, Shen SC. Propionibacterium acnes-induced iNOS and COX-2 protein expression via ROS-dependent NF-κB and AP-1 activation in macrophages. J Dermatol Sci. 2013; 69:122–131. PMID: 23178030.

27. Zhang H, Wang ZW, Wu HB, Li Z, Li LC, Hu XP, Ren ZL, Li BJ, Hu ZP. Transforming growth factor-β1 induces matrix metalloproteinase-9 expression in rat vascular smooth muscle cells via ROS-dependent ERK-NF-κB pathways. Mol Cell Biochem. 2013; 375:11–21. PMID: 23275087.

28. Ko HM, Koppula S, Kim BW, Kim IS, Hwang BY, Suk K, Park EJ, Choi DK. Inflexin attenuates proinflammatory responses and nuclear factor-kappaB activation in LPS-treated microglia. Eur J Pharmacol. 2010; 633:98–106. PMID: 20159010.
29. Lin HY, Tang CH, Chen YH, Wei IH, Chen JH, Lai CH, Lu DY. Peptidoglycan enhances proinflammatory cytokine expression through the TLR2 receptor, MyD88, phosphatidylinositol 3-kinase/AKT and NF-kappaB pathways in BV-2 microglia. Int Immunopharmacol. 2010; 10:883–891. PMID: 20451669.

30. Karunakaran S, Ravindranath V. Activation of p38 MAPK in the substantia nigra leads to nuclear translocation of NFkappaB in MPTP-treated mice: implication in Parkinson\'s disease. J Neurochem. 2009; 109:1791–1799. PMID: 19457134.
31. Manea A, Tanase LI, Raicu M, Simionescu M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2010; 396:901–907. PMID: 20457132.
32. Ago T, Kuroda J, Kamouchi M, Sadoshima J, Kitazono T. Pathophysiological roles of NADPH oxidase/nox family proteins in the vascular system. -Review and perspective-. Circ J. 2011; 75:1791–1800. PMID: 21673456.
33. Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996; 271:C1424–C1437. PMID: 8944624.

34. Gibbons HM, Dragunow M. Microglia induce neural cell death via a proximity-dependent mechanism involving nitric oxide. Brain Res. 2006; 1084:1–15. PMID: 16564033.

35. Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol. 2003; 14(8 Suppl 3):S211–S215. PMID: 12874433.

36. Hattori K, Naguro I, Runchel C, Ichijo H. The roles of ASK family proteins in stress responses and diseases. Cell Commun Signal. 2009; 7:9. PMID: 19389260.

37. Huo Y, Rangarajan P, Ling EA, Dheen ST. Dexamethasone inhibits the Nox-dependent ROS production via suppression of MKP-1-dependent MAPK pathways in activated microglia. BMC Neurosci. 2011; 12:49. PMID: 21615929.

38. Yang CS, Shin DM, Lee HM, Son JW, Lee SJ, Akira S, Gougerot-Pocidalo MA, El-Benna J, Ichijo H, Jo EK. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signalling. Cell Microbiol. 2008; 10:741–754. PMID: 18028450.

39. Yang CS, Lee HM, Lee JY, Kim JA, Lee SJ, Shin DM, Lee YH, Lee DS, El-Benna J, Jo EK. Reactive oxygen species and p47phox activation are essential for the Mycobacterium tuberculosis-induced pro-inflammatory response in murine microglia. J Neuroinflammation. 2007; 4:27. PMID: 18036262.

40. Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005; 102:5856–5861. PMID: 15809417.

41. Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007; 353:509–514. PMID: 17188246.

42. Boivin A, Pineau I, Barrette B, Filali M, Vallières N, Rivest S, Lacroix S. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci. 2007; 27:12565–12576. PMID: 18003835.

43. Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007; 282:14975–14983. PMID: 17355971.

Fig. 1
Dose determination for DEK treatment. (A) Cell viabilities were examined in BV-2 microglia using MTT assay to determine the safe and appropriate doses of DEK treatment. BV-2 cells were treated for 25 h with the indicated concentration of DEK. (B, C) Microglial cells were pretreated with DEK for 1 h and then stimulated with LPS (1 µg/ml) in the presence of DEK for 24 h. Effects of DEK on NO release were examined in culture media of LPS-stimulated primary microglial cells (B) and BV-2 microglial cell line (C) using Griess reagent. Values are the mean±S.E.M. of four samples in one independent experiment. The data were replicated in three repeated independent experiments. ###p<0.001 as compared to untreated control group and **p<0.01; ***p<0.001 as compared to LPS alone-treated group.
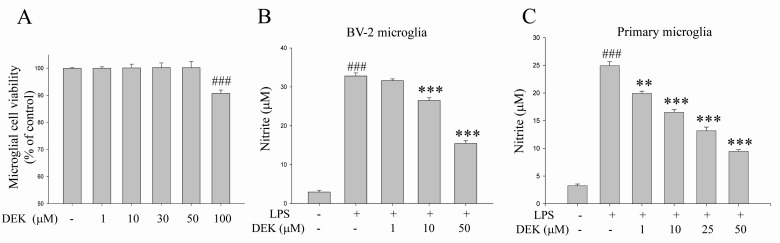
Fig. 2
Neuroprotective effects of DEK against microglial-mediated neuronal cell death. (A) BV-2 microglial cells were pretreated with DEK (50 µM) for 6 h. After wash-out, microglial cells were further incubated with LPS (1 µg/ml) for 24 h in the absence of DEK. MTT assay-based cell viabilities of HT-22 neurons were measured after different conditioned media treatment for 24 h. Groups are summarized as follows: the conditioned media from control BV-2 cells (Control-CM); LPS was added to the conditioned media from control BV-2 cells (Control CM+LPS); the conditioned media from LPS alone-treated BV-2 cells (LPS-CM); the conditioned media from LPS-treated BV-2 cells after DEK pretreatment (LPS/DEK-CM). (B) BV-2 microglial cells were pretreated with DEK (50 µM) for 6 h and then washed. B35-EGFP neurons were added to these BV-2 microglial cells to construct neuron-microglia co-culture system. Then, LPS (1 µg/ml) was stimulated for 24 h in the absence of DEK. The numbers of B35-EGFP neuronal cells were assessed by counting EGFP-positive cells under a fluorescent microscope. Fluorescent images of five random fields per well were captured and counted. Values are the mean±S.E.M. of four samples in one independent experiment. The data were replicated in three repeated independent experiments. ###p<0.001 as compared to the untreated control group and ***p<0.001 as compared to LPS alone-treated group.
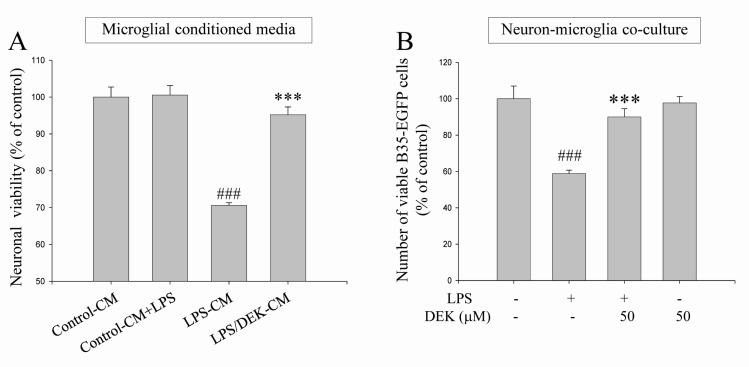
Fig. 3
Inhibitory effects of DEK on ERK phosphorylation in LPS-stimulated microglia. BV-2 microglial cells were pretreated with DEK (50 µM) for 1 h and then stimulated with LPS (1 µg/ml) in the presence of DEK for 30 min. Total proteins were subjected to SDS-PAGE, and probed with antibodies against phospho-ERK (A) and phospho-p38 (B) using Western blot analysis. Optical densities of individual protein bands were normalized to the corresponding levels of ERK and p38. Values are the mean±S.E.M. of four samples in one independent experiment. The data were replicated in three repeated independent experiments. #p<0.05, ###p<0.001 as compared to untreated control group and *p<0.05; ***p<0.001 as compared to LPS alone-treated group.
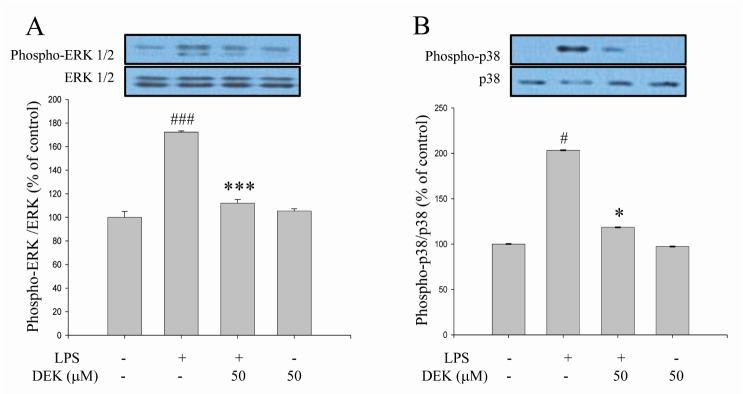
Fig. 4
Inhibitory effects of DEK on Akt-mediated pathways in LPS-stimulated microglia. BV-2 microglial cells were pretreated with DEK (50 µM) for 1 h and then stimulated with LPS (1 µg/ml) in the presence of DEK for 30 min. Total proteins were subjected to SDS-PAGE, and probed with antibodies against phospho-Akt. (A) Optical densities of individual protein bands were normalized to the corresponding levels of Akt. Levels of NF-κB p65 subunit in nuclear fraction (B) and IκBα in cytosol fraction (C) were determined using Western blot analysis. Optical densities of individual protein bands were normalized to the corresponding levels of β-actin or housekeeping nuclear protein TATA-binding protein (TBP). Values are the mean±S.E.M. of four samples in one independent experiment. The data were replicated in three repeated independent experiments. #p<0.05, ##p<0.01; ###p<0.001 as compared to untreated control group and *p<0.05; **p<0.01 as compared to LPS alone-treated group.
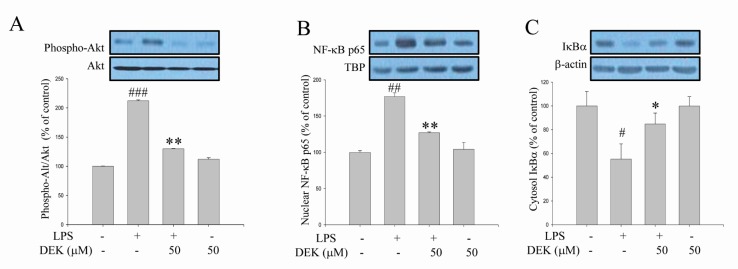
Fig. 5
Inhibitory effects of DEK on gp91phox expression in LPS-stimulated microglia. (A) The time course of LPS-induced expression of gp91phox, a catalytic component of NADPH oxidase complex, was investigated. BV-2 microglial cells were stimulated with LPS (1 µg/ml) for different time periods. Thereafter, protein fractions were extracted and subjected to Western blot analysis with anti-gp91phox antibody. (B) BV-2 cells were pretreated with DEK (50 µM) for 1 h and then stimulated with LPS (1 µg/ml) in the presence of DEK for 6 h, based on the time course data. Thereafter, protein fractions were extracted and subjected to Western blot analysis to investigate the effect of DEK on LPS-induced gp91phox expression. (C) BV-2 microglial cells were pretreated with apocynin and DPI, as NADPH oxidase inhibitors, for 1 h and then stimulated with LPS (1 µg/ml) in the presence of apocynin and DPI for 24 h. The amount of NO released in culture media was examined using Griess reagent to investigate effects of NADPH oxidase inhibitors on LPS-induced NO release. (D) Free radical scavenging activity of DEK was performed in cell-free system using DPPH assay. (E) The time course of LPS-induced intracellular ROS levels was investigated by spectrofluorometer using ROS-sensitive fluorescent dye DCF-DA. (F) BV-2 cells were pretreated with DEK (1, 10 and 50 µM) for 1 h and then stimulated with LPS (1 µg/ml) in the presence of DEK for 30 min. The intracellular ROS levels were determined by spectrofluorometer using ROS-sensitive fluorescent dye DCF-DA. Optical densities of individual protein bands were normalized to the corresponding levels of β-actin. Values were expressed as mean±S.E.M. #p<0.05, ##p<0.01 ###p<0.001 as compared to untreated control group and *p<0.05 **p<0.01; ***p<0.001 as compared to LPS alone-treated group.
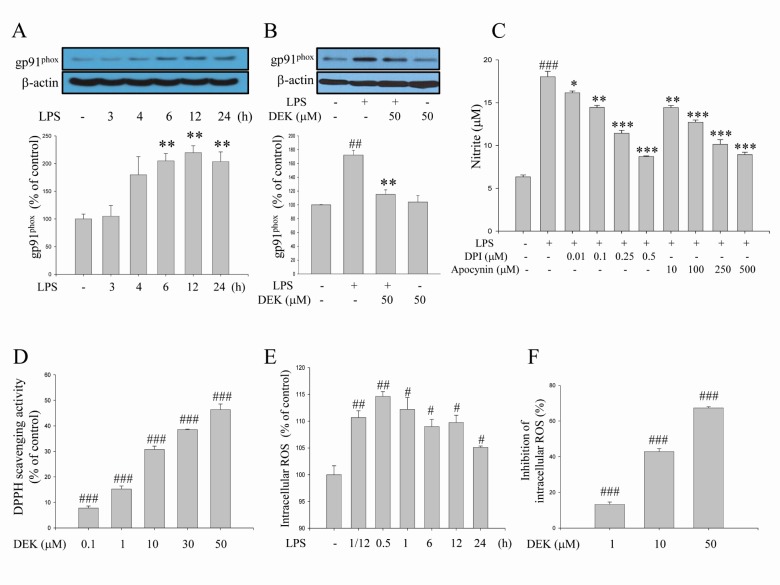
Fig. 6
Neuroprotective effects of the blockade of PI3K/Akt and NADPH oxidase against microglia-mediated neuronal cell death. Neuroprotective effects of the blockade of PI3K/Akt and NADPH oxidase were investigated against microglia-mediated neuronal cell death in microglial conditioned media system (A) and in neuronmicroglia co-culture system (B). Wortmannin (100 nM), LY294002 (25 µM) and apocynin (500 µM) were pretreated in both experimental systems and then, neuronal cell deaths were evaluated as shown in Fig. 2 (See 'Materials and methods' for the detailed description). Values are the mean±S.E.M. of four samples in one independent experiment. The data were replicated in three repeated independent experiments. ###p<0.001 as compared to untreated control group and **p<0.01 ***p<0.001 as compared to LPS alone-treated group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download