INTRODUCTION
METHODS
Reagents and cell culture
Cell viability
Nitrite quantification assay
Western blot analysis
ELISA assay for cytokines
Statistical analysis
RESULTS
1-p-Coumaryol β-D-glucoside (CG) suppresses NO and PGE2 production in LPS-stimulated RAW264.7 macrophage cells
 | Fig. 2CG attenuated LPS-induced NO and PGE2 production in RAW264.7 macrophage cells. RAW264.7 cells were pretreated with various indicated concentrations of CG for 1 hr, then incubated with LPS (200 ng/ml) for 24 hrs. The concentrations of NO (A) and PGE2 (B) in the supernatants were measured by Griess and ELISA assay as described in the methods. CG exhibited a significant suppression of LPS-induced NO (A) and PGE2 (B) secretion in concentration-dependent manners. No significant cell death was observed at CG concentrations used in the present study (C). Methyl p-hydroxycinnamate (MH) was used as a reference compound compared with CG. The data are expressed as mean±SD for three (NO) or four (PGE2) independent experiments. *p<0.05 and **p<0.01 indicate statistically significant differences from treatments with LPS alone. |
CG inhibits LPS-induced protein expression of iNOS and COX-2
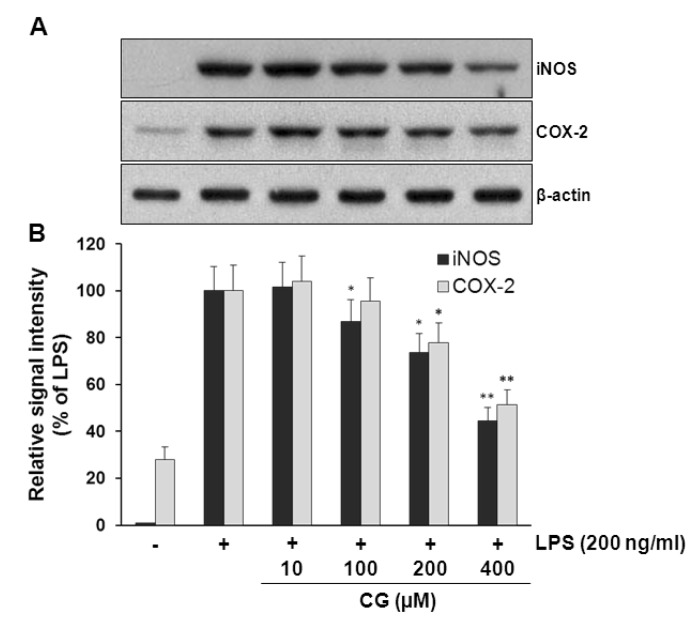 | Fig. 3CG inhibited LPS-induced iNOS and COX-2 protein expressions in RAW264.7 macrophage cells. (A) RAW264.7 cells were pretreated with indicated concentrations of CG for 1 hr, then incubated with LPS (200 ng/ml) for 24 hrs. The cell lysates were prepared and subjected to Western blotting analysis by using antibodies specific for iNOS and COX-2 as described in the methods. (B) The relative protein levels were quantified by scanning densitometry and normalized to β-actin. The data indicated that CG significantly decreased the LPS-induced overexpression of iNOS and COX-2 in a concentration-dependent manner. The images shown are representatives of three independent experiments that showed consistent results and the relative protein values are expressed as mean±SD for three experiments. *p<0.05 and **p<0.01 indicate statistically significant differences from treatments with LPS alone. |
CG attenuates LPS-induced release of pro-inflammatory cytokines such as IL-1β and TNF-α
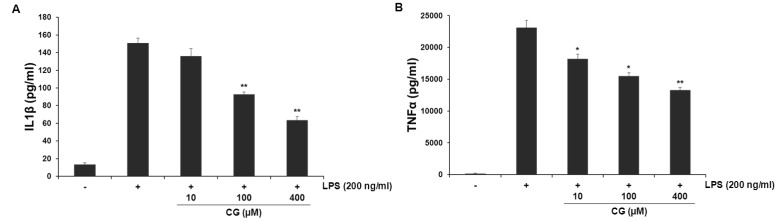 | Fig. 4CG suppressed the extracellular release of LPS-induced IL-1β and TNF-α cytokines in RAW264.7 macrophage cells. RAW264.7 cells were pretreated with indicated concentrations of CG for 1 hr, then incubated with LPS (200 ng/ml) for 24 hrs. The concentrations of IL-1β (A) and TNF-α (B) in collected cell culture media were measured by ELISA assay as described in the methods. CG meaningfully reduced LPS-stimulated both IL-1β and TNF-α cytokines in a concentration-dependent manner. The values are expressed as mean±SD for three independent experiments. *p<0.05 and **p<0.01 indicate statistically significant differences from treatments with LPS alone. |
CG attenuates LPS-induced degradation of IκB
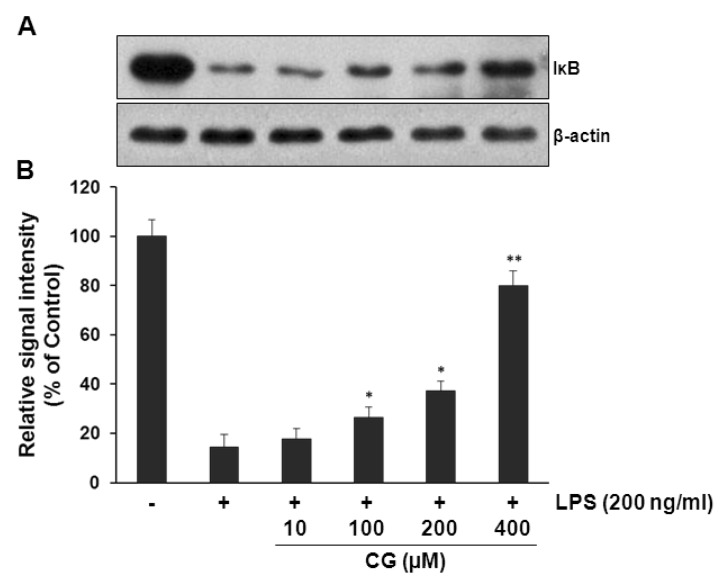 | Fig. 5CG significantly attenuated LPS-induced IκBα degradations in RAW264.7 macrophage cells. (A) RAW264.7 cells were pretreated with indicated concentrations of CG for 1 hr, then exposed to LPS (200 ng/ml) for 15 mins. The cell lysates were prepared and subjected to Western blotting analysis by using antibodies specific for IκB as described in the methods. (B) The relative protein levels were quantified by scanning densitometry and normalized to β-actin. The data showed that CG significantly prevented LPS-induced IκB degradations in a concentration-dependent manner. The images shown are representatives of three independent experiments that showed consistent results and the relative protein values are expressed as mean±SD for three experiments. *p<0.05 and **p<0.01 indicate statistically significant differences compared with LPS alone. |
Akt signaling pathway mediates the anti-inflammatory activity of CG
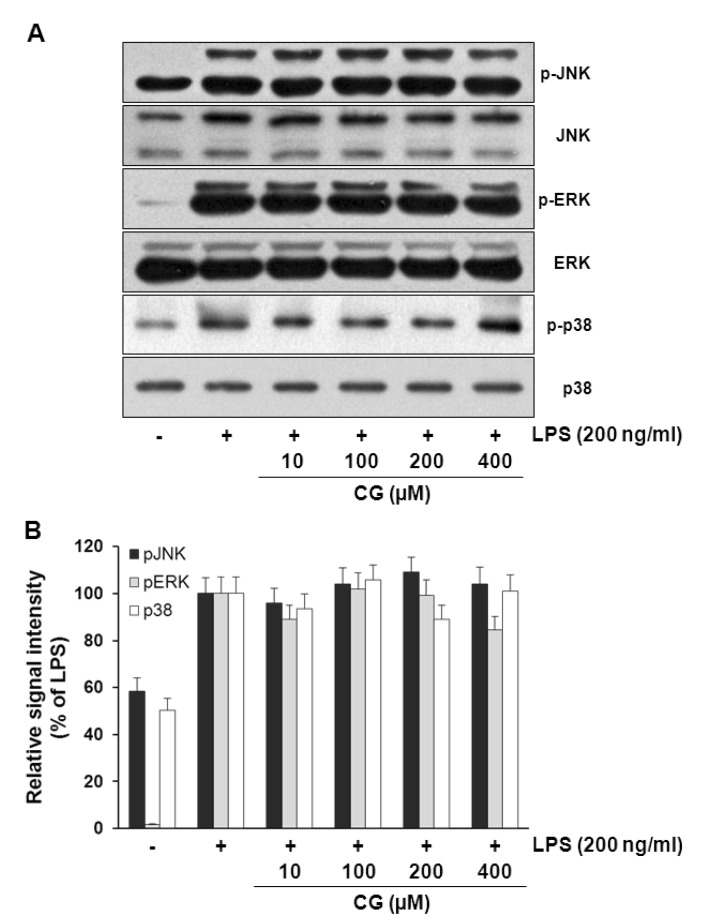 | Fig. 6CG did not affect MAPKs signaling pathways in RAW264.7 macrophage cells. (A) RAW264.7 cells were pretreated with various indicated concentrations of CG for 1 hr, then exposed to LPS (200 ng/ml) for 30 mins. The cell lysates were prepared and subjected to Western blotting analysis by using antibodies specific for total and phosphorylated forms (shown as p-) of JNK, ERK and p38. (B) The relative protein levels of p-JNK, p-ERK and p-p38 were quantified by scanning densitometry and normalized to total JNK, ERK and p38, respectively. The images shown are representatives of three independent experiments that showed consistent results and the relative protein values are expressed as mean±SD for three experiments. The data presented no effect of CG on MAPKs. |
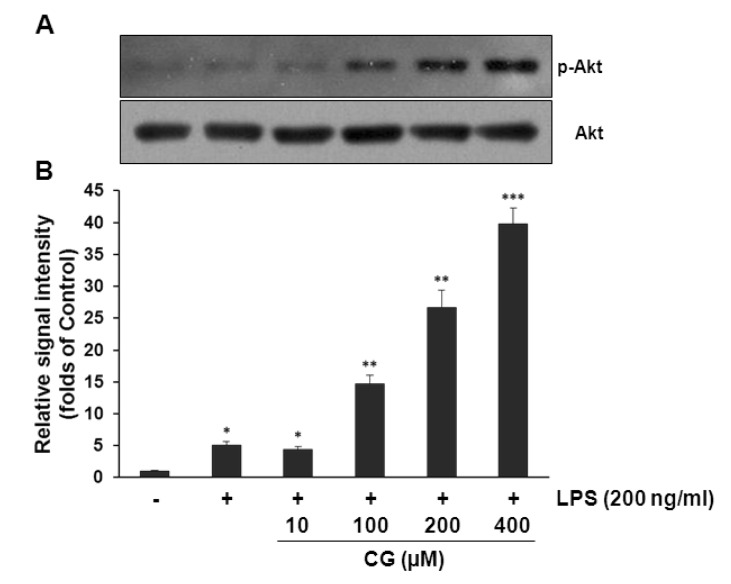 | Fig. 7CG significantly increased Akt phosphorylation in RAW264.7 macrophage cells. (A) RAW264.7 cells were pretreated with various indicated concentrations of CG for 1 hr, then exposed to LPS (200 ng/ml) for another 1 hr. The cell lysates were prepared and subjected to Western blotting analysis by using antibodies specific for total and phosphorylated forms of Akt. (B) The relative protein levels of p-Akt were quantified by scanning densitometry and normalized to total Akt. The data exhibited that CG strongly increased Akt activation in a concentration-dependent manner. The images shown are representatives of three independent experiments that showed consistent results and the relative protein values are expressed as mean±SD for three experiments. *p<0.05, **p<0.01, ***p<0.001 indicate statistically significant differences from treatments with control alone. |
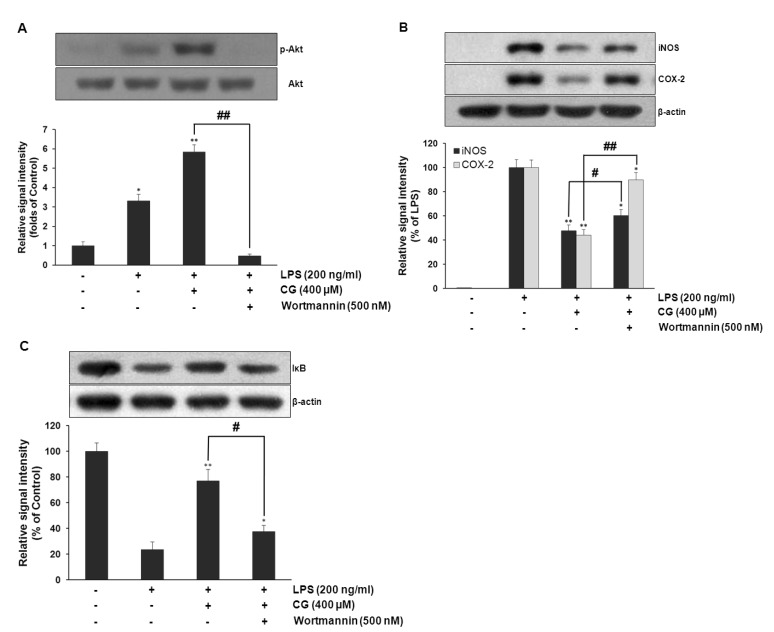 | Fig. 8PI3K/Akt was required for CG-mediated suppression of LPS-induced iNOS and COX-2 expressions and restoration of LPS-induced IκB degradation in RAW264.7 macrophage cells. RAW264.7 cells were pretreated with CG (100 µM) for 1 hr in the presence or absence of wortmannin, then exposed to LPS (200 ng/ml) for 1 hr. The cell lysates were prepared and subjected to Western blotting analysis by using antibodies specific for total and phosphorylated forms of Akt, which showed that wortmannin completely abolished CG-mediated Akt activation (A). The relative protein levels of iNOS and COX-2 were also examined. CG-mediated suppression of LPS-induced iNOS and COX-2 expressions were significantly attenuated with wortmannin but not completely (B). The protein level of IκB was examined. CG-mediated recovery of LPS-induced IκB degradation was significantly abolished with wortmannin (C). The images shown are representatives of three independent experiments that showed consistent results and the relative protein values are expressed as mean±SD for three experiments. *p<0.05, **p<0.01 indicate statistically significant differences from treatments with control alone. #p<0.05 and ##p<0.01 represent statistical significances between indicated groups. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download