INTRODUCTION
METHODS
shRNA construction and transfection
Determination of cell growth
Cell viability assay
Gene expression assays
Western blot analysis
Detection of nuclear NK-κB
Determination of cytokine production
RESULTS
Bcl-2-shRNA suppresses Bcl-2 mRNA and protein expression in Jurkat T cells
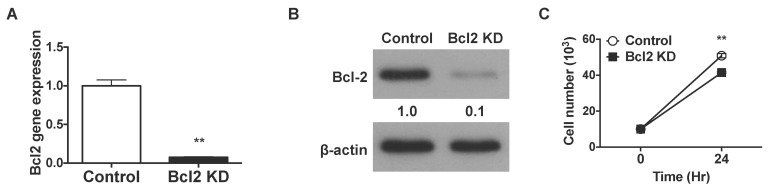 | Fig. 1shRNAs knock down Bcl-2 in Jurkat T cells. The bcl-2 gene is knocked down using shRNA in Jurkat T cells. (A) Total RNA was extracted from Bcl-2-knockdown and control Jurkat T cells and bcl-2 gene expression was analyzed by real-time PCR using GAPDH as the internal control. (B) Total protein was extracted from Bcl2-knockdown and control Jurkat T cells. Bcl-2 protein expression was analyzed by western blotting using β-actin expression as a loading control. (C) A total of 1×105 Jurkat T cells were seeded in a 96-well plate and incubated for 24 h. After incubation, 0.4% Trypan Blue was added to the cell suspension, and cell numbers were estimated by counting under a microscope. Cells stained blue were considered non-viable. Data were presented as mean±SD for triplicate determinations. Student's t test; *p<0.05; **p<0.01; and ***p<0.001 vs. control sample. All data were representative of at least three individual experiments. |
Bcl-2 knockdown increases TCR-triggered AICD and downregulates FLIP gene expression
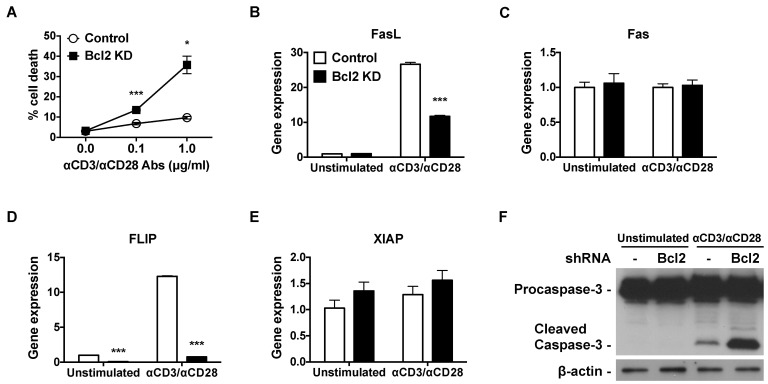 | Fig. 2Bcl-2 knockdown increases TCR-triggered AICD, downregulates FLIP gene expression, and upregulates caspase-3 cleavage. (A) Bcl-2-knockdown and control Jurkat T cells were incubated with 0.1 µg/ml or 1 µg/ml of plate bound anti-CD3 and anti-CD28 antibody for 24 h. Cells were washed with PBS, resuspended in PBS containing 5 µg/ml of PI, and analyzed by flow cytometry. (B~E) Bcl2-knockdown and control Jurkat T cells were incubated with 1 µg/ml of plate-bound anti-CD3 and anti-CD28 antibodies for 6 h. Total RNA was isolated, reverse transcribed, and gene expression was analyzed by real-time PCR. Relative gene expression levels were normalized with respect to those of GAPDH. (F) Bcl2-knockdown and control Jurkat T cells were incubated with 1 µg/ml of plate-bound anti-CD3 and anti-CD28 antibodies for 24 h. Total protein was extracted, and procaspase-3 and cleaved caspase-3 protein expression was analyzed by western blot. β-actin protein expression was used as a loading control. Data were presented as mean±SD for triplicate determinations. Student's t test; *p<0.05; **p<0.01; and ***p<0.001 vs. control sample. All data were representative of at least three individual experiments. |
Bcl-2 knockdown enhances the expression of TNFR and suppresses TRAF gene expression
 | Fig. 3Bcl-2 knockdown enhances the expression of TNFR and suppresses TRAF gene expression. Bcl-2-knockdown and control Jurkat T cells were incubated with 1 µg/ml of plate-bound anti-CD3 and anti-CD28 antibodies for 6 h. Total RNA was extracted and reverse transcribed, and gene expression was analyzed by real-time PCR. Relative gene expression levels of (A) TNFR1, (B) TNFR2, (C) TRAF3, (D) TRAF4 were normalized to those of GAPDH used as the internal control. Data were presented as mean±SD for triplicate determinations. Student's t test; *p<0.05; **p<0.01; and ***p<0.001 vs. control sample. All data were representative of at least three individual experiments. |
Bcl-2 knockdown suppresses the nuclear translocation of NF-κB
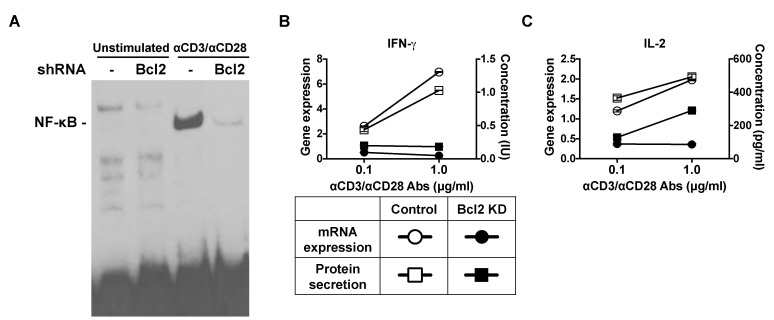 | Fig. 4Bcl-2 shRNA suppresses the nuclear translocation of NF-κB. (A) Bcl-2-knockdown and control Jurkat T cells were incubated with 1 µg/ml of plate-bound anti-CD3 and anti-CD28 antibodies for 30 min. Nuclear extracts were prepared and analyzed by using an electrophoretic mobility shift assay (EMSA). (B, C) Bcl-2 knockdown and control Jurkat T cells were incubated with 1 µg/ml of plate-bound anti-CD3 and anti-CD28 antibodies. After 24 h, supernatants were collected and analyzed for cytokines by using the enzyme-linked immunosorbent assay (ELISA) method. After 6 h, total RNA was isolated and reverse transcribed, and gene expression was analyzed by real-time PCR. Relative gene expression levels were normalized to those of GAPDH used as the internal control. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download