INTRODUCTION
METHODS
Reagents
Cell Preparation
Cell Culture
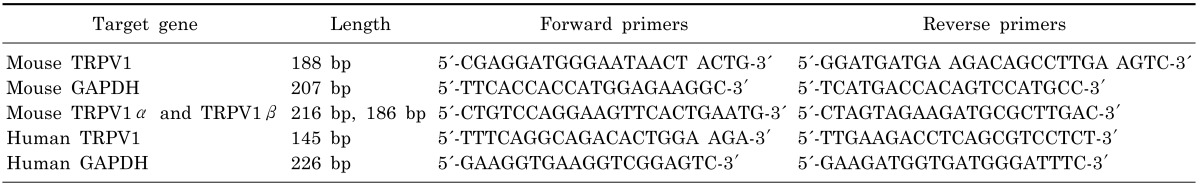
Reverse-transcriptase-polymerase chain-reaction
Quantitative Real-time PCR
Western Blotting
Immunofluorescence
Measurement of intracellular free calcium concentration ([Ca2+]i)
Saliva Collections
Transepithelial electrical resistance (TER) measurement
Statistics
RESULTS
Expression of TRPV1 in mouse and human salivary glands
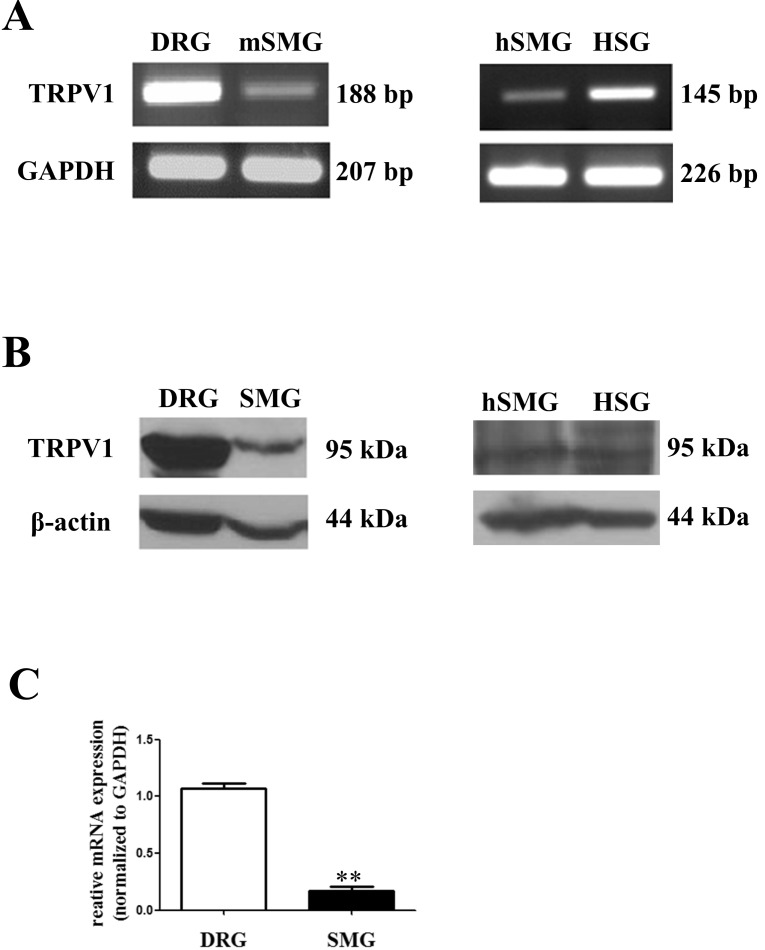 | Fig. 1Expression of TRPV1 in salivary gland epithelial cells (SGEC). (A) Expression of TRPV1 mRNA in mouse SMG (mSMG), human SMG (hSMG), and HSG cells by RT-PCR, with GAPDH as an internal control. (B) Expression of TRPV1 protein in mSMG, hSMG, and HSG cells by Western blot. Approximately 50 µg of protein was separated on 8% SDS-polyacrylamide gel electrophoresis and TRPV1-immunoreactivity was determined using a polyclonal anti-TRPV1 antibody. The blot is representative of five separate experiments with similar results. (C) Quantitative analysis of TRPV1 between DRG and SMG in mice by real-time PCR. TRPV1 mRNA levels are represented as ratios to the mRNA level of the housekeeping gene, GAPDH. Results are presented as mean±SD (n=4, **p<.001). |
Distribution of TRPV1 in mouse and human salivary glands
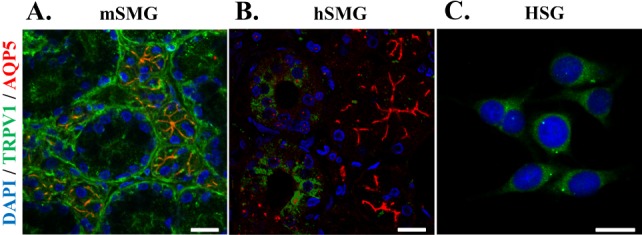 | Fig. 2Distribution of TRPV1 in salivary gland epithelial cells (SGEC). Representative immunofluorescence labeling of TRPV1 in mSMG (A), hSMG (B), and HSG cells (C). SMGs sections and HSG were immunostained with anti-TRPV1 antibody and AQP5 antibody, then incubated with Alexa Fluor-linked anti-rabbit IgG (red) and Alexa Fluor-linked anti-goat IgG (green). Nuclei (blue) were labeled with 4,6-diamidino-2-phenylindole. (A) TRPV1were mainly detected in apical membrane in acinar cells and in basolateral membrane in ductal cells. AQP5 was used as a marker for acinar cells that was exclusively expressed in the apical membrane of acinar cells (red color). The merged orange color indicates that expression of TRPV1 at the apical membrane in acinar cells. (B) In hSMG, higher expression levels of TRPV1 was observed in ductal cells compared to acinar cells. (C) In HSG cells, TRPV1 was widespread in the cytoplasm. Scale bar indicates 20 µm. The results are representative of four independent experiments. |
Calcium response of TRPV1 to capsaicin in mouse and human salivary glands
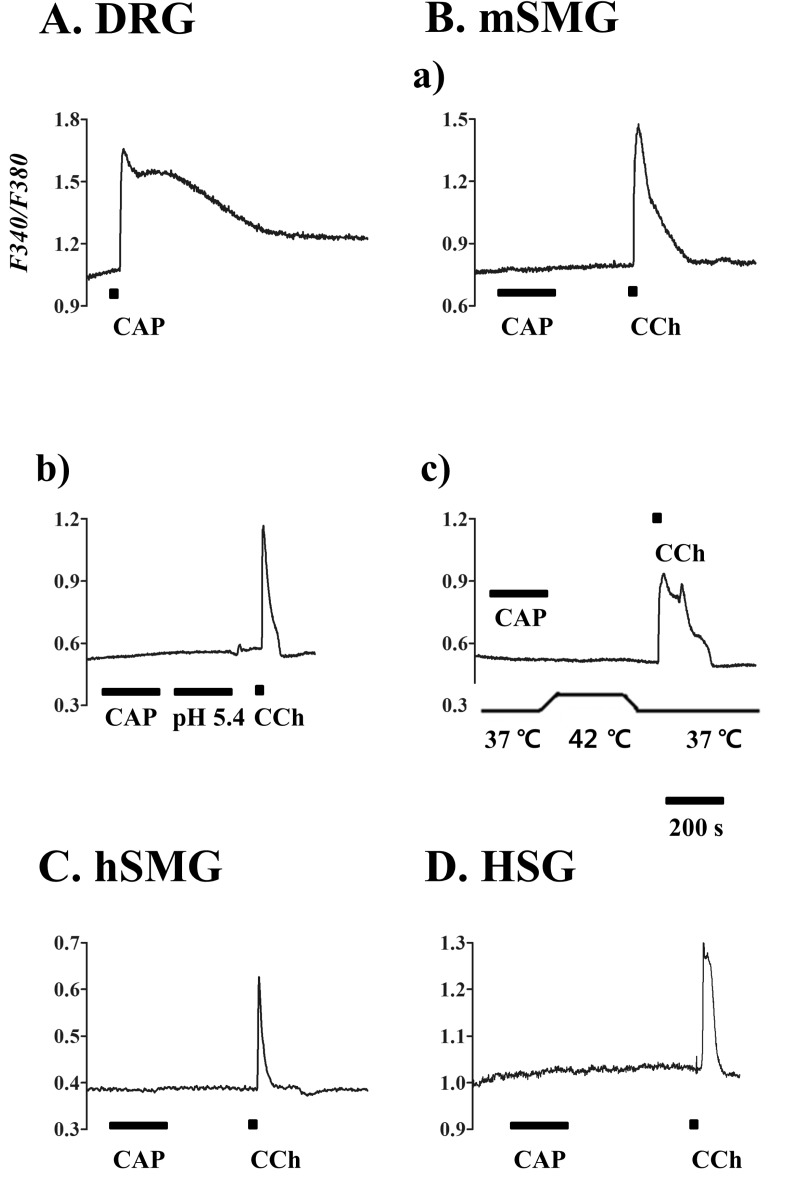 | Fig. 3Calcium response to capsaicin in salivary gland epithelial cells (SGEC). [Ca2+]i responses induced by capsaicin (CAP) in mouse dorsal root ganglion (DRG) (A), mSMG (B), hSMG (C), and HSG cells (D). (A) CAP 1 µM in DRG as positive control. (B) CAP 10 µM (a), acidic solution with pH 5.4 (b), and hot temperature of 42℃ (c) in the acinar cells from mSMG. CAP 10 µM in the acinar cells from hSMG (C) and HSG cells (D). It is of note that 10 µM carbachol (CCh) applied at the end of each experiment consistently increased [Ca2+]i. Data for each trace were obtained from ≥30 cells in at least 5 separate experiments. |
Effect of capsaicin on salivary secretion and TRPV1 splice variants expression
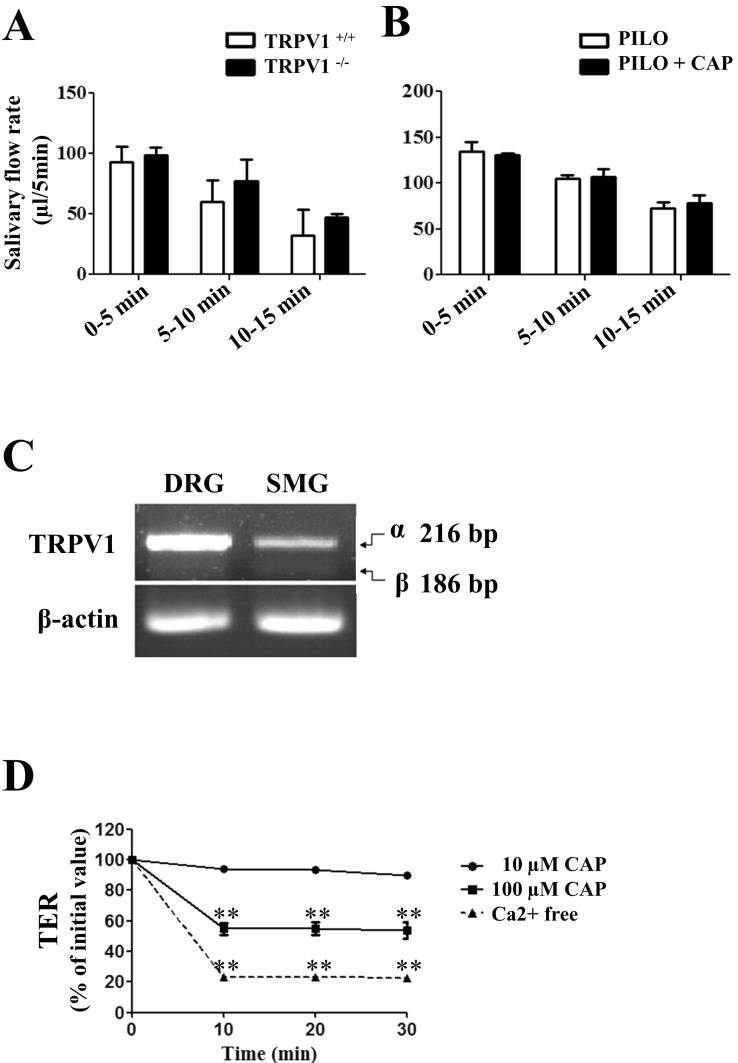 | Fig. 4Effect of capsaicin on salivary secretion and TRPV1 splice variants expression. (A) To measure the whole saliva volume, pilocarpine (PILO) was administered by i.p. injection in wild-type and TRPV1 knock-out mice. (B) PILO alone (white columns) or PILO+CAP (dark columns) were administered by i.p. in wild-type mice. Data are shown as mean±SD for collections made on four mice in each group. Statistically insignificant differences are shown between columns. (C) Expression patterns of α and β isoforms of TRPV1 in mouse DRG and mSMG by RT-PCR. The mRNA transcripts of 216 bp and 186 bp corresponding to TRPV1 isoforms were detected. The upper band represents TRPV1α, while lower band denotes TRPV1β. Data are representative of four independent experiments. (D) Decrease of paracellular permeability in SMG-C6 cells by CAP application. Time course of TER was measured using an EVOM. Although 10 µM CAP had no effect on TER values, the high concentration of CAP, 100 µM, evoked a significant decrease in TER from 10 to 30 min (n=3, **p<.001). Incubation of cells in a Ca2+-free medium, as a positive control, caused a decrease in TER values from 10 to 30 min (n=3, **p<.001). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download