INTRODUCTION
METHODS
Slice preparation
Electrophysiological recording
Chemicals
Statistical analysis
RESULTS
Calcium- and CaMKII-mediated regulation of phasic inhibition
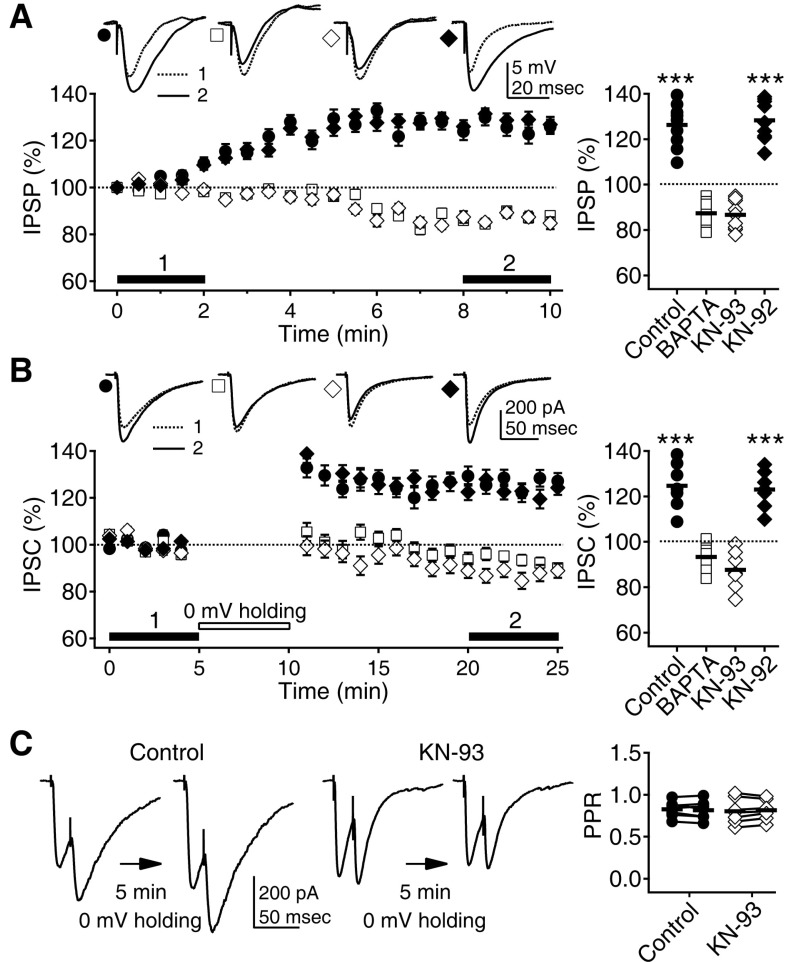 | Fig. 1Depolarization-induced potentiation of phasic inhibition depends on Ca2+ and CaMKII. (A) IPSPs increased with time at a 0 mV membrane potential (solid circle). IPSPs were recorded with K-gluconate-based pipette solution in the presence of the AMPA, NMDA, and GABAB receptors blockers DNQX (20 µM), D-AP5 (50 µM), and CGP 52432 (1 µM) and were evoked with electrical stimulation. The inclusion of the Ca2+-chelator BAPTA (10 mM, open square) and the CaMKII inhibitor KN-93 (10 µM, open diamond) in the pipette solution even depressed the IPSPs. However, KN-92 (10 µM, solid diamond), an inactive analog of KN-93, did not affect the depolarization induced potentiation of IPSPs. Left panel shows the time courses of changes in IPSPs while the membrane potential was held at 0 mV. Insets show representative traces of averaged IPSPs at the indicated period. Right panel plots individual data (symbols) and averages (thick solid lines). ***p<0.001 vs. BAPTA or KN-93. (B) Five min of 0 mV holding potentiated IPSCs recorded with CsCl-based pipette solution at a -75 mV holding potential in the presence of DNQX, D-AP5, and CGP 52432 (closed circle). BAPTA (open square) and KN-93 (open diamond) blocked the potentiation. KN-92 (solid diamond) had no effect. Left panel shows the time courses of changes in IPSCs. Insets show representative traces of averaged IPSCs at the indicated period. (C) PPR was not affected by the 5min of 0 mV holding, suggesting postsynaptic changes. Paired responses were 20 msec apart. Left panels show representative traces of paired IPSCs before and after the 0 mV holding. Right panel plots individual data showing PPRs before and after the 0 mV holding (symbols linked by lines) and averages (thick solid lines). |
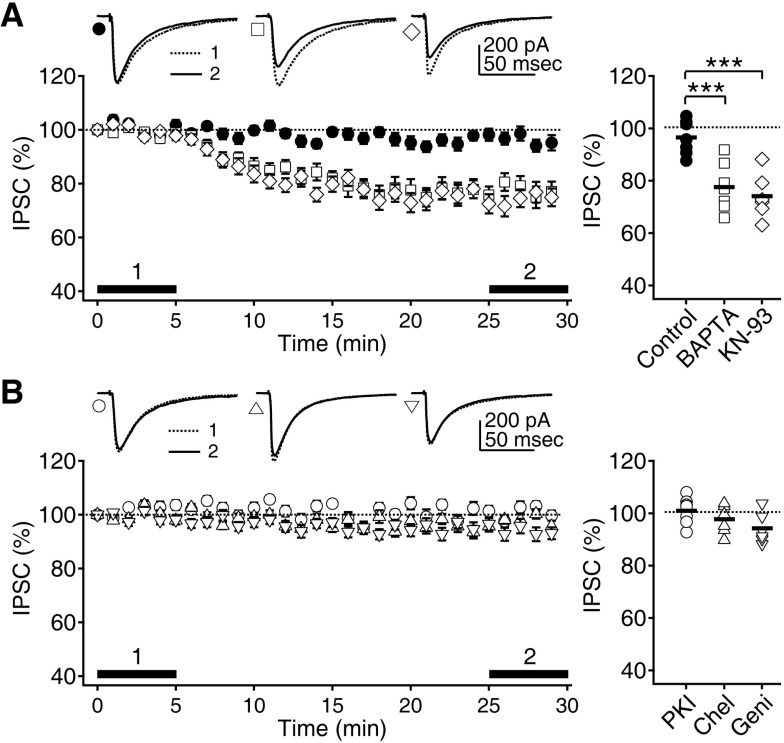 | Fig. 2Maintenance of phasic inhibition depends on Ca2+ and CaMKII. (A) IPSCs recorded with CsCl-based pipette solution at a -75 mV holding potential were well maintained for at least 30 min (closed circle). Inclusion of BAPTA (open square) and KN-93 (open diamond) in the pipette solution decreased IPSCs with time. Left panel shows the time courses of changes in IPSCs. Insets show representative traces of averaged IPSCs at the indicated period. Right panel plots individual data (symbols) and averages (thick solid lines). ***p<0.001 between groups linked by line. (B) Inclusion of the PKA, PKC, and tyrosine kinases inhibitors PKI (100 µg/ml, open circle), chelerythrine (50 µM, 'Chel', open triangle), and genistein (100 µM, 'Geni', open inverse triangle) in the pipette solution did not affect the IPSCs. |
Maintenance of tonic inhibition by the activity of PKA
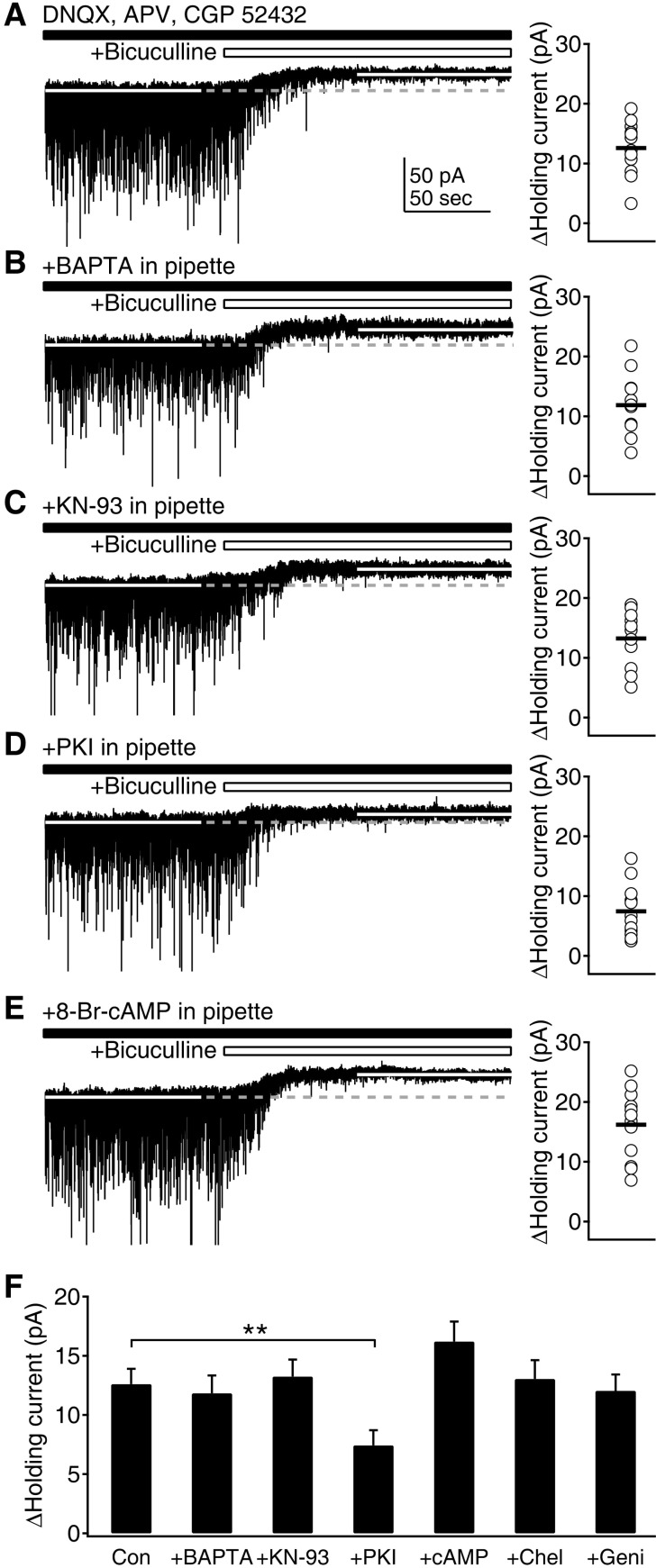 | Fig. 3Maintenance of tonic inhibition depends on cAMP and PKA. (A) Tonic GABAAR-mediated currents were measured as changes in holding currents by the application of the GABAAR blocker bicuculline (10 µM) at a -75 mV holding potential in the presence of DNQX, D-AP5, and CGP 52432. Left panel shows a representative trace of current recording to measure the tonic current. Right panel plots individual data (symbols) and averages (thick solid lines). (B) Measurement of tonic currents with BAPTA in the pipette solution. (C) Measurement of tonic currents with KN-93 in the pipette solution. (D) Measurement of tonic currents with PKI in the pipette solution. (E) Measurement of tonic currents with the PKA activator 8-Br-cAMP (10 µM) in the pipette solution. (F) Average tonic currents were plotted for control condition ('Con'), BAPTA, KN-93, PKI, 8-Br-cAMP ('cAMP'), chelerythrine ('Chel'), and genistein ('Geni') in the pipette solution. **p<0.01 between groups linked by line. |
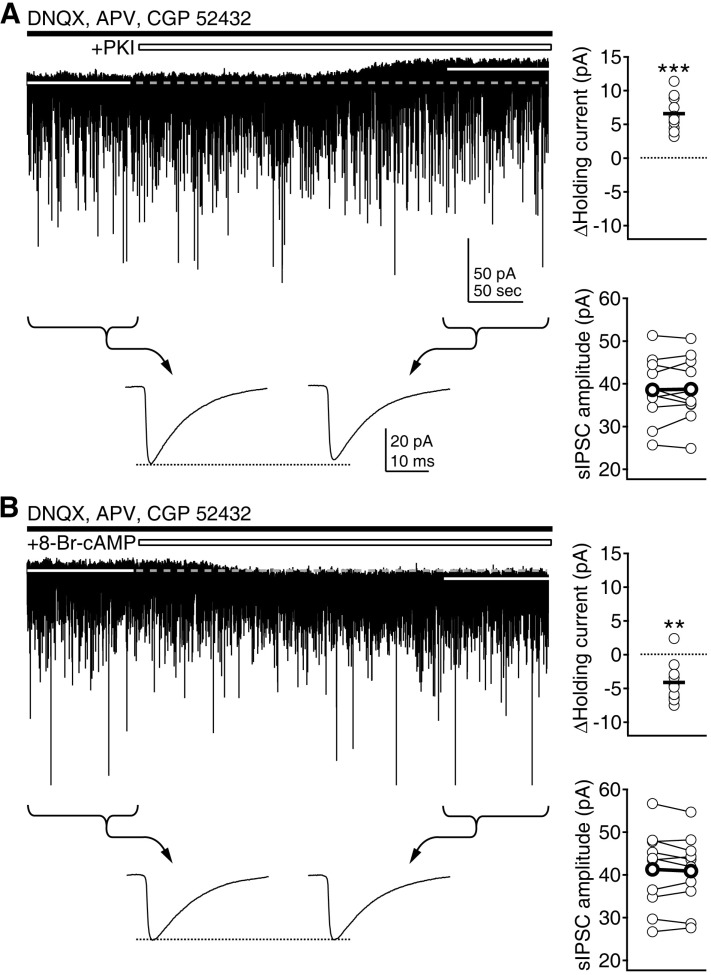 | Fig. 4Regulation of tonic inhibition by PKA. (A) Cell-permeable myristoylated PKI (1 µM) was applied during the continuous recording of currents. Changes in holding currents and the amplitude of sIPSCs were investigated. Left upper panel shows a representative trace of current recording. Average amplitude of sIPSCs for 100 sec period before and after the application of drug was compared (indicated by braces and arrows in lower traces, showing averaged sIPSCs at the indicated period). Right panels plot individual data (symbols and thin symbols linked by lines) and averages (thick solid lines and thick symbols linked by lines). ***p<0.001 vs. baseline. (B) 8-Br-cAMP (10 µM) was applied during the continuous recording of currents. **p<0.01 vs. baseline. |
Independent regulation of phasic and tonic inhibition
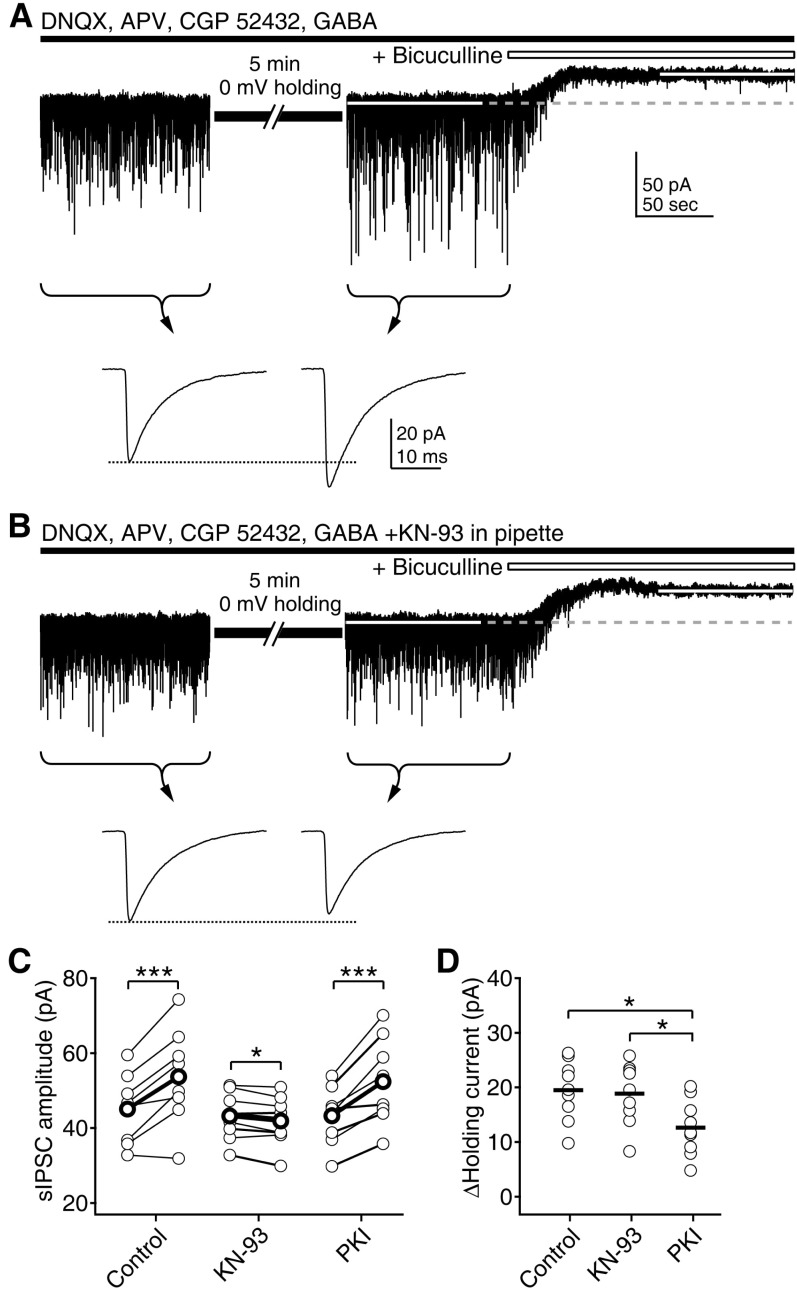 | Fig. 5Depolarization selectively enhances phasic inhibition via CaMKII. (A) Selective enhancement of sIPSCs by 5min of 0 mV holding. After stabilizing the current recording at a -75 mV holding potential, membrane potential was held at 0 mV for 5 min. Average amplitude of sIPSCs for 100 sec period before and after the depolarization was compared (indicated by braces and arrows in lower traces, showing averaged sIPSCs at the indicated period). Then, bicuculline was applied to measure tonic GABAAR-mediated currents. (B) The inclusion of KN-93 in the pipette solution inhibited the enhancement of sIPSCs. (C) Individual data of changes of sIPSC before and after the depolarization (thin symbols linked by lines) and averages (thick symbols linked by lines) were plotted for the control condition, KN-93 and PKI in the pipette solution. *p<0.05, ***p<0.001 between the sIPSCs before and after depolarization. (D) Individual data (symbols) and averages (thick solid lines) of tonic currents measured after the depolarization. *p<0.05 between groups linked by line. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download