INTRODUCTION
METHODS
Reagents used
Preparation of human peripheral neutrophils
Preparation of mouse bone marrow neutrophils
Preparation of mouse peripheral neutrophils
Preparation of human monocytes and lymphocytes
Cell culture
Etheno-NAD cleaving activity
HPLC
Statistical analysis
RESULTS
Comparison of extracellular etheno-NAD cleaving activity of intact human peripheral neutrophils with other immune cell types
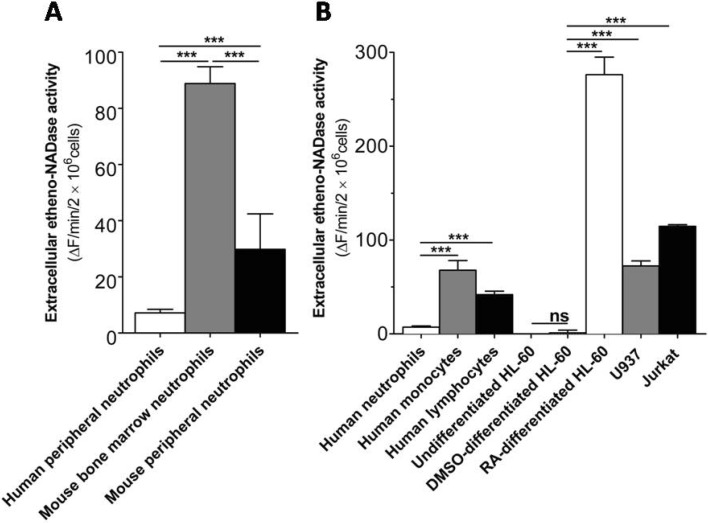 | Fig. 1Intact human peripheral neutrophils show much less extracellular etheno-NAD cleaving activity than other immune cell types. (A) Comparison of extracellular etheno-NAD cleaving activity of human peripheral neutrophils with mouse bone marrow neutrophils and mouse peripheral neutrophils. (B) Comparison of extracellular etheno-NAD cleaving activity of human peripheral neutrophils with other primary immune cells and comparison of neutrophil-like cells with other immune cell lines. Briefly, each cell type was suspended in HBSS and seeded to 96 well-plate at a cell density of 1×107 cells/ml. Substrate etheno-NAD (final concentration 20 µM) was added following 5 min pre-read. Cleavage of etheno-NAD was continuously followed for 15 min as described in Materials and Methods. Activity was defined as the fluorescence change (▴F)/min/2×106 cells. ns, no significant difference; ***p<0.0001. |
Identification of the major extracellular metabolite generated from extracellular NAD degradation by human peripheral neutrophils and mouse bone marrow neutrophils
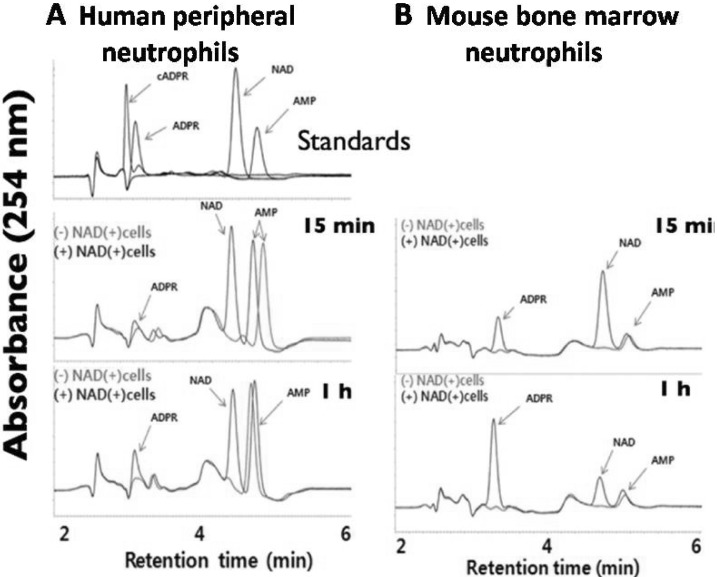 | Fig. 2ADPR is the major extracellular metabolites generated from the degradation of NAD by both intact human peripheral neutrophils and mouse bone marrow neutrophil as determined by HPLC. Human peripheral neutrophils at a density of 1×107 cells/ml (A) and mouse bone marrow neutrophils at a density of 3×106 cells/ml (B) were incubated with or without NAD (1 µM) and incubated for 15 min or 1 hour at 37℃. Then the extracellular media were collected after centrifugation. Aliquots were analyzed by reverse-phase HPLC (Jasco Instruments) as described in Methods. |
N-Formyl-methionine-leucine-phenylalanine (fMLP) downregulates extracellular etheno-NAD cleaving activity of intact human neutrophils
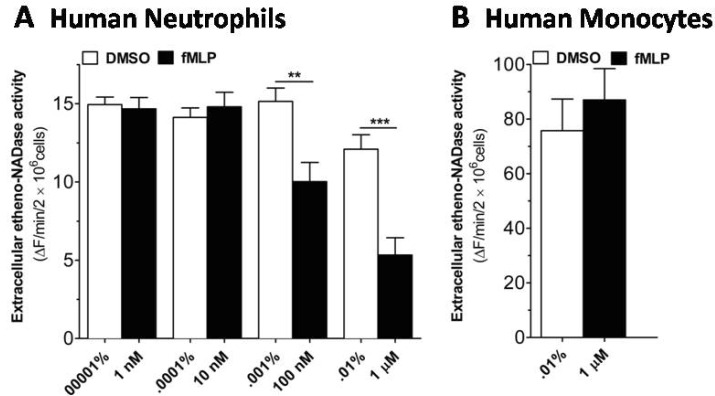 | Fig. 3fMLP induces a decrease in extracellular etheno-NAD cleaving activity of human neutrophils, but not of human monocytes. (A) Human neutrophils and (B) human monocytes were suspended in HBSS and seeded to 96 well-plate at a cell density of 1×107 cells/ml and incubated in the CO2 humidified chamber for 10 min. Then vehicle or fMLP of different concentration was treated. Substrate etheno-NAD (final concentration 20 µM) was added following 5 min pre-read. Cleavage of etheno-NAD was continuously followed at 37℃ for 15 min. Activity was defined as the fluorescence change (▴F)/min/2×106 cells. **p<0.001; ***p<0.0001. |
WRW4, an FPRL1 antagonist, blocks fMLP-induced decrease in the extracellular etheno-NAD cleaving activity of intact human neutrophils
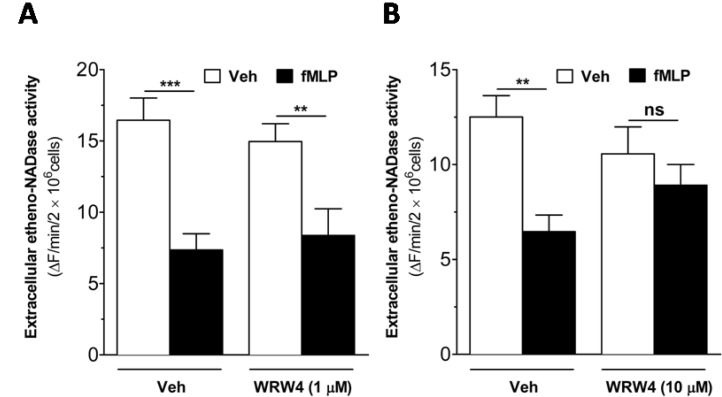 | Fig. 4fMLP-induced decrease in extracellular etheno-NAD cleaving activity of intact human neutrophils is reversed by WRW4, an FPRL1 antagonist. Human neutrophils were suspended in HBSS and seeded to 96 well-plate at a cell density of 1×107 cells/ml. Then, the cells were treated with vehicle or WRW4 (1 µM (A) or 10 µM (B)) for 5 min before the treatment of vehicle or fMLP (1 µM). Substrate etheno-NAD (final concentration 20 µM) was added following 5 min pre-read. Cleavage of etheno-NAD was continuously followed at 37℃ for 15 min. Activity was defined as the fluorescence change (▴F)/min/2×106 cells. ns, no significant difference; **p<0.001; ***p<0.0001. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download