ABBREVIATIONS
BM
MLCK
DP
CP
PMLC20
TTX
RLCS
RHCS
ACh
SNP
L-NNA
ICCs
NC
INTRODUCTION
METHODS
Animals
Drugs
Experimental models of diarrhea and constipation
Tissue preparation and contractility determination
Jejunal contractility measured in different contractile states
Western blot analysis
PT-PCR analysis of myosin light chain kinase mRNA expression
Statistical analysis
RESULTS
Concentration-effects relationship of diprophylline
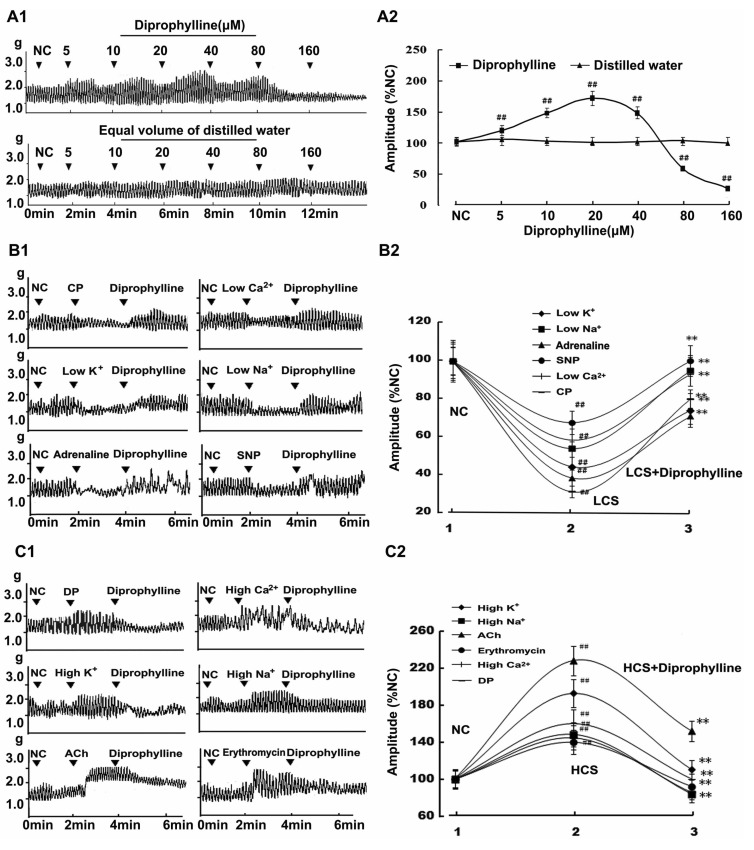 | Fig. 1Diprophylline-induced bidirectional modulation (BM) on jejunal contractility. (A1) Representative traces and (A2) statistical analysis of concentration-response relationship for diprophylline-indued effects on jejunal contractility. (B1) Representative traces and (B2) statistical analysis of diprophylline-exerted stimulatory effects on the contractility of jejunal segments in 6 low contractile states (LCS). (C1) Representative traces and (C2) statistical analysis of diprophylline-exerted inhibitory effects on the contractility of jejunal segments in 6 high contractile states (HCS). Contractile amplitude of jejunal segment in normal contractile state is set to 100% (NC). Data are expressed as the mean±SEM (% NC, n=6). ##p<0.01 compared with NC. **p<0.01 compared with the contractile amplitudes in LCS and the contractile amplitudes in HCS before diprophylline treatment respectively. CP, constipation-prominent; DP, diarrhea-prominent; SNP, sodium nitropr usside; ACh, acetylcholine. |
Relationship between jejunal contractile state and diprophylline-induced effects
Underlying mechanisms involved in diprophylline-induced bidirectional modulation
1. Links between enteric nervous system and diprophylline-induced bidirectional modulation
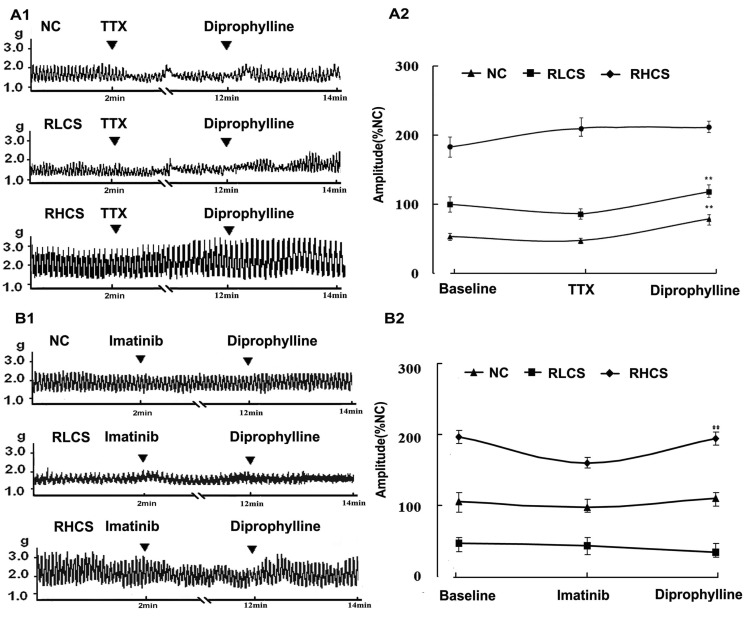 | Fig. 2Effects of diprophylline on jejunal contractility pretreated with tetrodotoxin (TTX) and imatinib respectively. (A1) Representative traces and (A2) statistical analysis of diprophylline (20 µM)-induced effects on jejunal segment pretreated with tetrodotoxin (0.1µM). (B1) Representative traces and (B2) statistical analysis of diprophylline on the contractility of jejunal segment pretreated with c-Kit tyrosine kinase blocker imatinib. Contractile amplitude of jejunal segment in normal contractile state is set to100% (normal control, NC). Data are expressed as the mean±SEM (% NC, n=6). **p<0.01 compared with contractile amplitude of jejunal segment after treatment with TTX or imatinib. RLCS, representative low contractile state; RHCS, representative high contractile state. |
2. Links between pacemaker activity of interstitial cells of Cajal and diprophylline-induced bidirectional modulation
3. Links between Ca2+ and diprophylline-induced bidirectional modulation
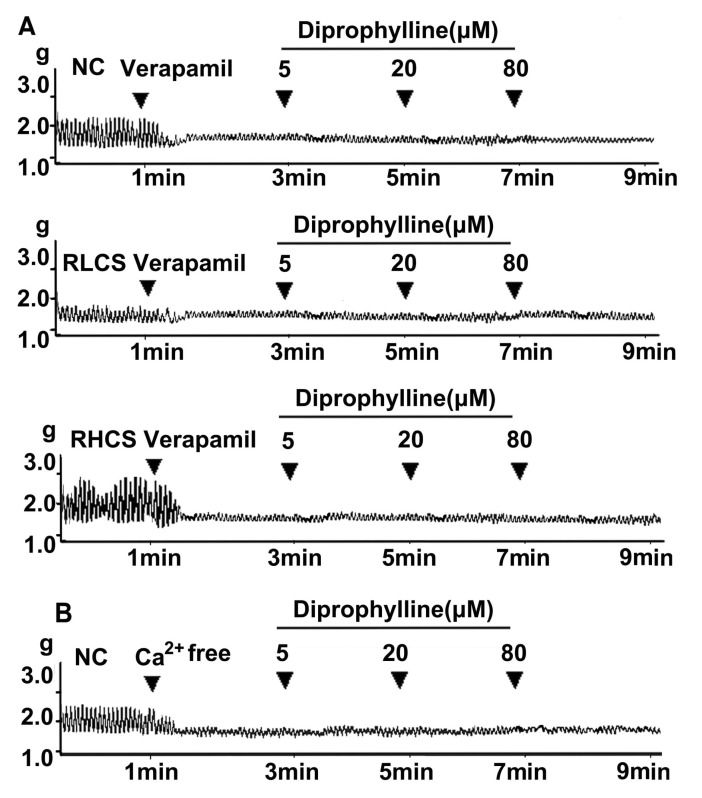 | Fig. 3Links between Ca2+ and diprophylline-induced effects. (A) Representative traces of diprophylline (5~80 µM)-induced effects on the contractility of jejunal segment pre-treated with verapamil (0.1 µM) in normal contractile state (NC), representative low contractile state (RLCS) and representative high contractile state (RHCS). (B) Representative traces of the effects of diprophylline (5~80 µM) on the contractility of jejunal segment assayed in Ca2+-free Krebs buffer. |
4. Receptors and enzymes potentially involved in diprophylline-induced bidirectional modulation
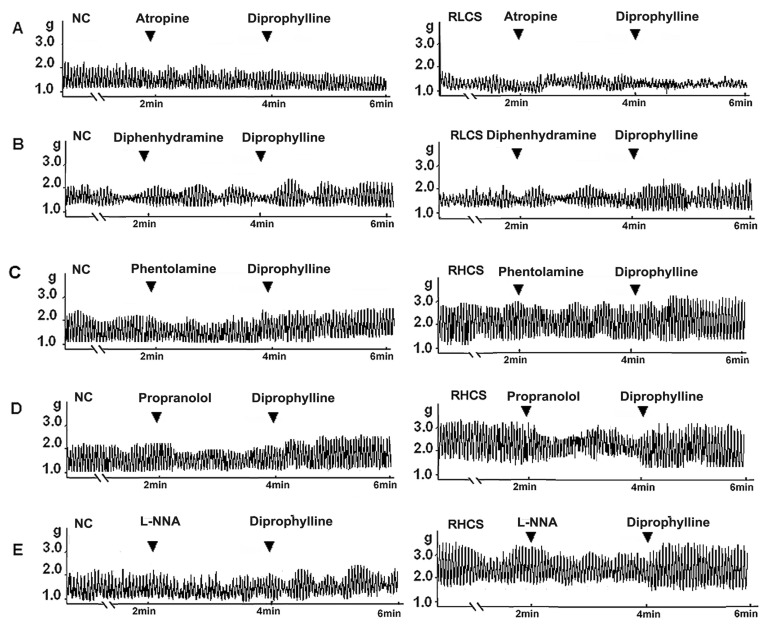 | Fig. 4Receptors and enzymes potentially involved in diprophylline-induced bidirectional modulation. Effects of diprophylline on the jejunal contractility pretreated with (A) 10 µM atropine and (B) 10 µM diphenhydramine respectively in normal contractile state (NC) and in representative low contractile state (RLCS). Effects of diprophylline on jejunal contractility, pretreated with (C) 10 µM phentolamine, (D) 5 µM propranolol, and (E) 10 µM L-NG-nitroargenine (L-NNA) respectively in NC and representative high contractile state (RHCS). |
5. Diprophylline-induced bidirectional modulation on myosin-phosphorylation
 | Fig. 5Effects of diprophylline on myosin phosphorylation. (A1) Representative traces and (A2) statistical analysis of the phosphorylation of 20-kDa regulatory light chain of myosin (p-MLC20). (B1) Representative traces and (B2) statistical analysis of the protein content of myosin light chain kinase (MLCK). (C) Statistical analysis of the expression of MLCK mRNA. Data obtained from normal control (NC) is set to 100%. Data are expressed as the mean±SEM (% NC, n=6). **p<0.01 compared with the NC. ##p<0.01 compared with the jejunal contractility before diprophylline treatment. CP, Constipation-prominent; DP, diarrhea-prominent; DIP, diprophylline. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download