INTRODUCTION
Diabetes mellitus is characterized by peripheral neuropathy of sensory and motor nerves. Peripheral nerves and muscles dysfunctions have been demonstrated in both humans and rodents [
1,
2,
3]. The morphological destabilization of neuromuscular junction (NMJ) in diabetes such as axonal degeneration, axonal atrophy and demyelination was has been verified [
4,
5].
It has been reported that the hyposensitivity to
d-Tubocurarine and other non-depolarizing muscle relaxants (NDMRs) in diabetic state [
6,
7]. However, the hypersensitivity to acetylcholine (ACh) and succinylcholine due to the decrease of ACh release and the augmentation of presynaptic acetylcholine receptors (AChRs) sensitivity was has been investigated [
8,
9]. The profound response to muscle relaxants in diabetes needs further investigation.
Mammalian skeletal muscle fibers are commonly divided into three types: slow-oxidative (type I), fast-glycolytic (type IIb), and fast-oxidative-glycolytic (type IIa). The typing of muscle fibers is based upon oxidative and glycolytic capacities, twitch characteristics, and ATPase activity [
10]. Extensor digitorum longus muscle (EDL), and soleus muscle (SOL) are typical fast and slow twitch muscles, composed of predominately fast and slow twitch fibers respectively. Many previous studies have focused on the effects of diabetes on the muscle contractile properties, which depends on the alteration of muscle types [
11]. The fiber-type switching from fast to slow isoforms was manifested in diabetes rodents [
12]. Regarding the contractile properties, an increase in the contraction and relaxation times in SOL in part reflecting a shift in the isomyosin composition with diabetes, which was indicated by an increased percentage of histochemically demonstrable slow fibers, at the expense of fast fibers, was testified. Moreover, a decrease in tetanic tension in EDL with no effect on muscle strength and performance, which was relative to atrophy of the fast-glycolytic fibers normally making the largest contribution to tension, was proved [
13].
Findings of previous studies have indicated the differences in muscle relaxation in slow and fast twitch muscles. It has been reported that type I muscle fiber is more resistant to NDMRs [
14,
15,
16,
17]. Motor neurons distributed to each muscle have muscle-specific characteristics that are reflected in differences in ACh release [
18]. The switching of muscle fiber types affects motor neurons and NMJs subsequently. NMJs at type I and IIa fibers are smaller and less complex compared to NMJs at type IIx and/or IIb fibers [
19]. The safety factor for neuromuscular transmission is higher in fast- than in slow-twitch muscles. In comparison with NMJs in slow-twitch muscles, those in fast muscles are reported to have a higher quantal content, more functional AChRs, a higher density of voltage-gated sodium channels in both junctional and extrajunctional regions. Postsynaptic folds are shorter, broader and sparser in slow-twitch muscles than in fast-twitch muscles [
20]. The fiber-type switching results in different performances of SOL and EDL in diabetes, which may underpin the mechanisms of different effects to NDMRs in these two muscles.
Whilst the contractile and histochemical properties of SOL and EDL have been studied extensively, little is known about the pharmacodynamics of muscle relaxants in those muscles. Of note the fact is that NMJs are the very early target in diabetes, the effects of diabetes on NDMRs actions in SOL and EDL may be changed [
5]. It is worth notice noticing that the progress in muscle functions and fiber alteration occurs in a time-dependent manner [
4]. It has not been investigated whether Whether the effects of diabetes on the actions of NDMRs depends on the stage of this disease or not has not been investigated.
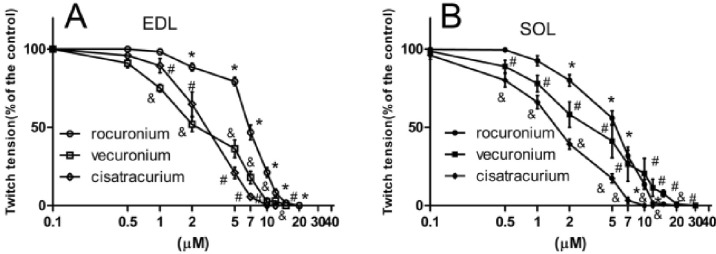
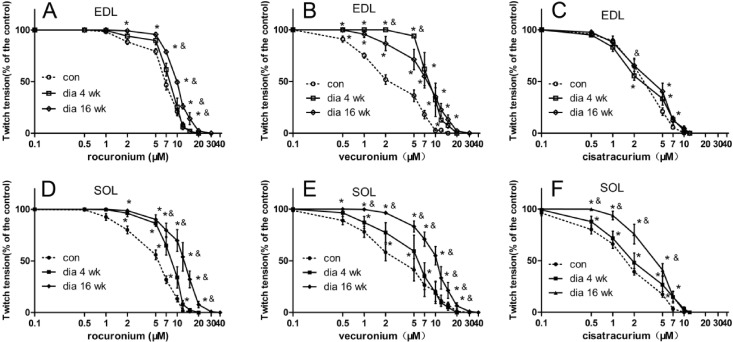
The aim of this study was to test the differences in the magnitude of resistance to vecuronium, cisatracurium, and rocuronium in SOL and EDL at different stages of streptozotocin (STZ)-induced diabetes. The hypothesis of this study was that diabetes-induced desensitization to NDMRs depends on the stage of diabetes, different muscles observed, and on the kind of NDMRs. We investigated the actions of vecuronium, cisatracurium, and rocuronium on twitch tensions of the EDL sciatic nerve-muscle preparations, and the SOL sciatic nerve-muscle preparations from rats after 4weeks and 16 weeks of STZ treatment.
Go to :

METHODS
Animals
The study was approved by the Animal Care Committee in Shanghai Jiaotong University (Shanghai, China). Forty male Sprague-Dawley rats (Experimental Animal Center of the School of Medicine, Shanghai Jiaotong University, Shanghai, China), weighing 200 to 240 g, were housed in groups of three. They were fasted but allowed to have free access to water and food prior to the experiments.
Induction of diabetes
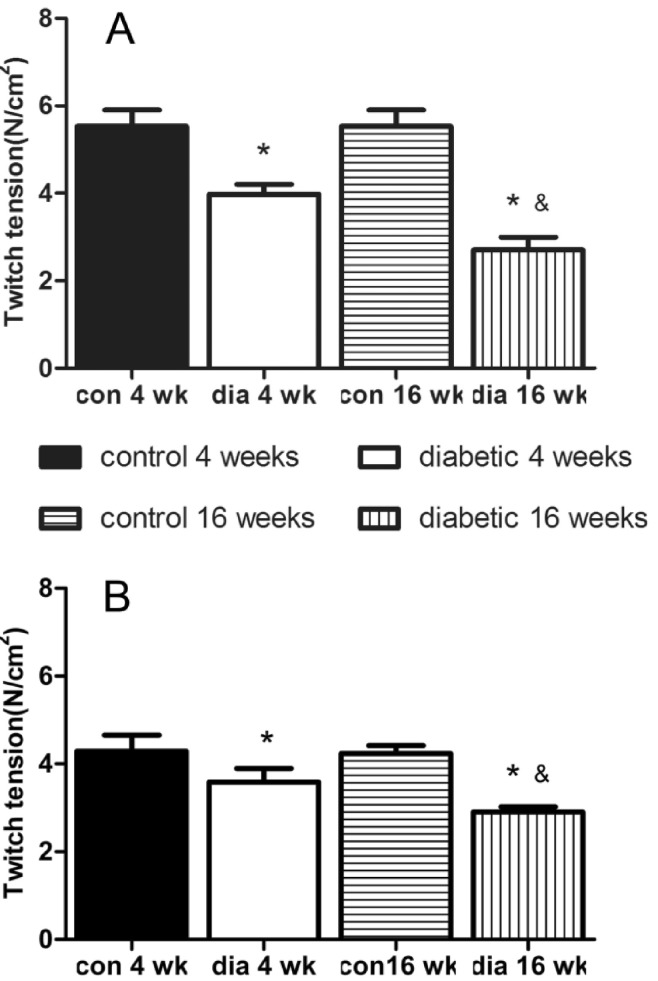
Rats were randomly divided into two groups. One group (n=20) was made diabetic group by a single injection of STZ 60 mg/kg intraperitoneally after 12 hours fasting. Another group (n=20) was given the same volumes of citric acid buffer solution, which was made up of normal animals matched in age. 2 days after injection, blood sugar was measured twice in series by cutting tails. Successful models were determined if the blood sugar values were above 16.7 mmol/L. The rats with blood sugar tested below 16.7 mmol/L were rejected from the study. After modeling successfully, the diabetic rats and the age-matched controls were randomly divided into two subgroups, diabetic 4 weeks group(dia 4 wk) and diabetic 16 weeks (dia 16 wk) group, control 4 weeks group (con 4 wk) and control 16 weeks group(con 16 wk) (n=10 each group)respectively. The rats were sacrificed at 4 and 16 weeks after STZ administration.
Nerve-muscle preparations
12 rats were killed with 60 mg/kg pentobarbital intraperitoneally. The isolated EDL sciatic nerve-muscle preparations and the SOL sciatic nerve-muscle preparations were established for indirectly electrical stimulation as described previously [
21,
22]. Body temperature was maintained at 37℃ using a heating blanket and radiant heat.
Either EDL or SOL was exposed in one leg. After measurements were completed for one muscle, the other was then exposed in the other leg. The SOL was exposed by sectioning the tendons connecting the plantaris and gastrocnemius muscles to the heel, and reflecting the muscles back. Silk thread was attached to the distal tendon of the SOL and the tendon was sectioned. The muscle was then carefully freed of surrounding tissues, ensuring the blood supply remained intact, and the sciatic nerve was sectioned. The EDL was prepared in the similar manner after first exposing the muscle by reflection of the anterior tibialis muscle. The silk sutures were tied to the proximal and distal tendons of the EDL and/or soleus muscles and the muscles were removed, tendon to tendon.
The isolated nerve-muscle preparations were dipped immediately into plexiglass chambers filled with Krebs solution, maintained at 37℃ and bubbled with 95% oxygen/5% CO2. The composition of the Krebs solution was as follows: 137 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1 mM KH2PO3, 12 mM NaHCO3 and 6.5 mM glucose, with a pH 7.40±0.05 during bubbling.
NDMRs potency
The indirect electrical stimulation-evoked twitch tension was recorded with MPA Multiple Channel Biological Signal Analysis System (provided by the Department of Anesthesiology, Shanghai First People's Hospital of SJTU). Each isolated strip was mounted vertically in a tissue chamber, inferiorly positioned. The EDL and SOL preparations were aligned vertically with distal tendon attached to the force displacement transducer (ALCM System for Isolated TissueOrgan Research; 40 ml in volume), proximal tendon fixed to the stainless steel fixed-post. The chamber was filled with Krebs solution as mentioned above. The nerves of the preparations were positioned on wire bipolar platinum electrodes for indirect stimulation. Isometric tension was elicited by indirect supramaximal constantvoltage stimulation at 0.1 Hz for 0.05 ms, using a stimulator and a constantvoltage unit. The twitch tension was recorded via the force transducer on a recorder (ALCMPA 2000m, Acquisition and Analysis System for Life Science Research, Shanghai Alcott Biotech, Shanghai, China). The stimulator was activated by a personal computer. Twitch stimuli were used to determine the optimal length (L0) at which skeletal muscle may generate the greatest force, followed by a 15 min thermo-equilibration period. A number of measurements were performed as described in subsequent sections.
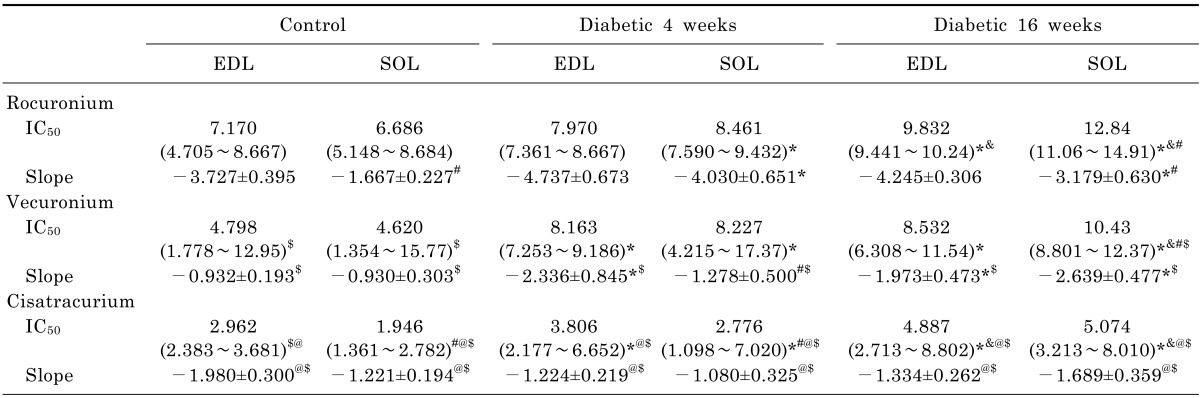
After the elicited twitch tension had been stabilized for a minimum of 15 min, the singletwitch tension, averaged in groups of five, was determined. The proximal end of the nerve attached to the strips was stimulated with a single supramaximal train of rectangular pulses (stimulation intensity which is the minimal stimulation intensity that can induce maximal force of contraction, duration 0.2 ms). The pulse was repeated three times at 5-second intervals, and the mean muscular tension amplitude was calculated. Before vecuronium, cisatracurium or rocuronium was added in, the baseline twitch tension amplitude was recorded as the control value. The NDMR was then applied to the preparation at the concentration of 0.01, 0.1, 0.5, 1, 2, 5, 7, 10, 12, 15, 20, 30, 40, 50µmol/l. Drug concentrations were determined by adding freshly prepared solutions with calibrated micropipettes to modified Krebs solution (40 ml) in the tissue chamber. Following stabilization of the drug effect for a minimum of 10 min, singletwitch tension was again determined. Data were accepted when twitch tension returned to 95~105% of the initial value by rinsing the diaphragm preparation with Krebs solution in each study. Concentration-twitch tension curves were constructed and the values of concentration giving 50% of maximal inhibition (IC50) were obtained. Rocuronium bromide and vecuronium were obtained from N.V. Organon (Oss, The Netherlands), while cisatracurium from GlaxoSmithKline (Italy). And all other drugs were purchased from SigmaAldrich (St. Louis, MO, USA).
Statistical analysis
Competition analysis data ([IC50] and slope at IC50) were determined from a four-variable logistic sigmoidal dose-response model fitted to the concentration-twitch tension curves (all values were considered for analysis) with the computer program Prism 4 (GraphPad Software, Inc., San Diego, CA). All other data were analyzed by SPSS version 13.0 software (IBM, Armonk, NY). Data are expressed as mean±SD. One- or two-way analysis of variance was used to test the significance of differences among all groups. The paired t test was used for comparison between any two groups. p<0.05 was considered to indicate a statistically significant difference.
Go to :

DISCUSSION
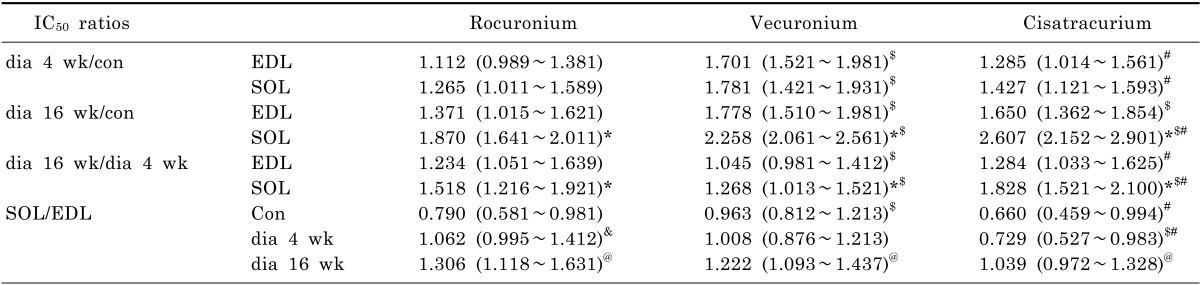
The results of this study demonstrated that diabetes-induced desensitization to NDMRs depended on the stage of diabetes and on the different kind of muscles observed while was independent on different kind of NDMRs.
In previous studies, the hyposensitivity to NDMRs in diabetes has been testified, which was consistent with our findings in this report. In this study, the concentration-twitch tension curves of rocuronium, vecuronium, and cisatracurium were significantly different in the diabetic groups compared with the control group. And the IC
50 was significantly larger in the diabetic groups than the control group. Nerve conduction velocity is reduced in diabetic peripheral neurons, and pathogenetic mechanisms responsible for the impairment of axons and Schwann cells have been examined [
4,
23]. Axonal atrophy and demyelination, which was the signs of nerve degeneration, and the destabilization at NMJ in diabetic state may be the most probable explanation [
4,
24]. Nevertheless, the decrease of ACh release and the augmentation of presynaptic AChRs sensitivity, which due to the hypersensitivity to depolarizing muscle relaxant succinylcholine, has been shown [
23,
25]. All these controversial preclinical results may be relevant to the findings in clinical practice, in which delayed recovery or no alteration was determined [
26,
27]. Although current findings of resistance to muscle relaxants in diabetic rats was not in accord with that of succinylcholine or in human beings, different kinds of muscle relaxants, varied experiment conditions,
in vivo or
in vitro, different species should not be neglected. The slopes of the concentration-twitch tension curves, which indicated the affinities of AChRs, were larger in diabetic groups than that in control group [
28]. The alteration in affinities of AChRs may occur in diabetic state. The similarity of nerve degeneration with denervation may induce the increased amount of AChRs. To the best of our knowledge, the distributions of muscle-type of AChRs are mainly restricted to the neuromuscular junction and activation of AChRs initiates contraction of skeletal muscle fibers by triggering endplate potentials at neuromuscular junctions [
29]. AChRs appear over the entire muscle fiber surface, and fetal type AChR is expressed throughout the muscle fiber after denervation [
30]. To achieve the same actions of neuromuscular blocking, the doses of NDMRs need to be increased to competitively bind to AChRs in diabetes. Further investigation should be done to testify the hypothesis.
Moreover, there was a significantly difference between the IC
50 in the dia 4 wk group and those in dia 16 wk group for rocuronium and cisatracurium in both SOL and EDL in this study. IC
50 was significantly larger in dia 16 wk group than those in the dia 4 wk group for vecuronium in SOL. Although there was no significantly difference between the IC
50 values in the dia 4 wk group and those in dia 16 wk group for vecuronium in EDL, IC
50 was larger in dia 16 wk group. In a word, regardless of the different muscle observed, diabetes induced the resistance to each NDMRs depends on the stage of diabetes for rocuronium, vecuronium and cisatracurium. The progress in muscle functions and fiber alteration occurs in a time-dependent manner [
4]. In Brotto et al's report, the alteration in diaphragm contractility depended on the time after the onset of STZ-induced diabetes, which at least in part, relied on the switch in fiber type [
12]. Although NMJs are the very early target in diabetes, the course of disease may play an important role in the final performance of changes in muscle contractility and alterations of response to NDMRs.
Compared with SOL in control group, IC
50 in EDL was larger for rocuronium, vecuronium and cisatracurium than that in EDL. While in diabetic groups, IC
50 in SOL was larger for every NDMRs. Additionally, IC
50 ratio of SOL/EDL in dia 16 wk group was larger than that in dia 4 wk group. The results meant that the response to NDMRs depended on the muscles observed. STZ diabetes had differential effects on skeletal muscles accompanying the course of diabetes. It has been shown that diabetic SOL muscles had a higher proportion of slow-oxidative fibers than weight-matched controls while no obvious changes in fiber composition in diabetic EDL muscles [
13]. For the slow SOL of the diabetic animals, muscle weight was preserved with respect to body weight. However, preferential atrophy of fast fibers in EDL was reflected in fiber area measurements [
28,
31]. Whereas SOL was protected from diabetic effects in terms of muscle mass and force production, both speed-related properties and oxidative capacity were impaired. In contrast, EDL underwent profound wasting and had decreased strength, while the speed-related properties and oxidative capacity was unchanged. The differences in the contractile and histochemical properties of slow and fast muscles may account for the differences in the resistance to NDMRs. The changes in fiber-type proportions of SOL which involved a change in muscle phenotypic expression, may induce more type I muscle fibers, which may result in much more resistance to NDMRs in SOL than that in EDL.
Compared with control rats, the IC
50 in diabetic groups were different among the neuromuscular blockers. In addition, the IC
50 ratios among the neuromuscular blockers were quite different with each other. However, there was no obvious relevance between the different kind of NDMRs and IC
50 ratios. E. Narimatsu et al. concluded that the magnitude of sepsis-induced attenuation of potencies to NDMRs depended on the different molecular structures of neuromuscular blockers [
28]. To the best of our knowledge, there are two kinds of NDMRs,: one is benzylisoquinolines (cisatracurium) and the other is bis-quaternary ammonium steroids (vecuronium and rocuronium). Our results demonstrated that diabetes-induced resistance to NDMRs was independent on the kind of NDMRs.
The results of our study seemed to be of great clinical relevance. Though the experiment was obtained in vitro by nerve-muscle preparations, the findings can be extrapolated to clinical studies. From the relevance in the conditions of different stages of diabetes between rat models and human beings, it is assumed that in clinical practice, the hyposensitivity to NDMRs may also develop to different extents during the course of diabetes. Moreover, now that diabetes-induced desensitization to NDMRs depended on the different kind of muscles observed, extra caution should be taken in clinical practice when monitoring for muscle relaxation in anesthetic management using different muscles. It is worth noticing that when choosing drugs for diabetic patients in anesthesia, NDMRs do not need further consideration as diabetes-induced resistance to NDMRs was independent on the kind of NDMRs.
In summary, diabetes-induced desensitization to NDMRs depended on the stage of diabetes and on the different kind of muscles observed while was independent on different kind of NDMRs. The resistance to NDMRs was stronger in the later stage of diabetes (16 weeks verus 4 weeks after STZ treatment in this report). Additionally, when monitoring in SOL, diabetes attenuated the actions of neuromuscular blockade than that in EDL. Nonetheless, the hyposensitivity to NDMRs in diabetes was not relevant for the kind of NDMRs.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download