Abstract
The purpose of the present study was to investigate cardiac damage biomarkers after a triathlon race in elite and non-elite athlete groups. Fifteen healthy men participated in the study. Based on performance, they were divided into elite athlete group (EG: n=7) and non-elite athlete group (NEG: n=8). Participants' blood samples were obtained during four periods: before, immediately, 2 hours and 7 days after finishing the race. creatine kinase (CK), creatine kinase-myoglobin (CK-MB), myoglobin, and lactate dehydrogenase (LDH) were significantly increased in both groups immediately after, and 2 hours after finishing the race (p<.05). CK, CK-MB, and myoglobin were completely recovered after 7 days (p<.05). Hematocrit (Hct) was significantly decreased in both groups (p<.05) 7 days after the race. LDH was significantly decreased in the EG (p<.05) only 7 days after the race. Homoglobin (Hb) was significantly decreased in the NEG (p<.05) only 2 hours after the race. Although cardiac troponin T (cTnT) was significantly increased in the EG but not in the NEG 2hours after the race (p<.05), there was no group-by-time interaction. cTnT was completely recovered in both groups 7 days after the race. In conclusion, cardiac damage occurs during a triathlon race and, is greater in elite than in non-elite. However, all cardiac damage markers return to normal range within 1 week.
Over the years, researchers have investigated to describe the changes in biomarkers of cardiac damage during recovery from strenuous and prolonged exercise events [1,2,3,4,5,6,7]. A triathlon is an endurance sport-event, which includes swimming, cycling and running with various distances. Olympic triathlon involving swim 1.5 km, bike 40 km, and run 10 km (total 51.5 km) and Ironman triathlon involving swim 3.8 km, cycle 180 km, and marathon 42 km (total 226 km) have recently become also popular in Korea.
Exercise-mediated physical treatment has attracted much interest in recent years. In particular, swimming and cycling are considered as effective exercise mode for patients with muscular and cardiovascular diseases [8]. However, triathletes may experience a variety of medical problems during training and competition, including hyponatremia [5], hemolysis [9], immunosuppression [10], and muscle damage [11].
Exercise-induced skeletal muscle damage is the most interesting adverse consequence in triathlon competition which is related with exercise performance and cardiac health [4,12,13]. Previous studies have found that serum creatine kinase(CK), hydroxybutyrate dehydrogenase(HBD), and lactate dehydrogenase (LDH) are traditional markers of myocardial damage in both pathological and physiological conditions [14,15]. However, recent studies have suggested that CK-MB isoenzyme, myoglobin, and cTnT are better markers of exercise-induced myocardial injury. Creatine isoenzymes (CK-MB, CK-MM) are markers of muscle and liver damage. Troponins(cTnI, cTnT) are markers that are used in a clinical setting to detect minor cardiac damage [11].
It has been demonstrated that strenuous, long-lasting exercise is associated with an increase in biomarkers of myocardial damage such as cTnT even in healthy trained subjects without signs of myocardial disease [13,16]. The reason why apparently healthy subjects exhibit increased concentrations of cTnT is not yet fully understood. The amounts and release time of plasma biochemical markers of muscle damage rely on the type, intensity, level of exercise, and the time of recovery [17].
Although previous studies revealed that increased level of the biochemical indexes of muscle injury following strenuous exercise, especially marathon running [11,15,17,18] or ultra-triathlon [7] such as Ironman triathlon, less is revealed about the acute changes of these indexes in elite and non-elite triathletes following Olympic triathlon competition.
The different performance level in athlete and the different distance of competition may also be valuable, as a multi-sport events such as triathlon may cause more cardiac muscle damage compared to the individual events such as swimming, cycling and running, and therefore, the comparisons between these different events may be inappropriate.
Accordingly, the aim of present study was to examine the effects of Olympic triathlon on specific cardiac biomarkers in elite and non-elite triathletes following a triathlon competition.
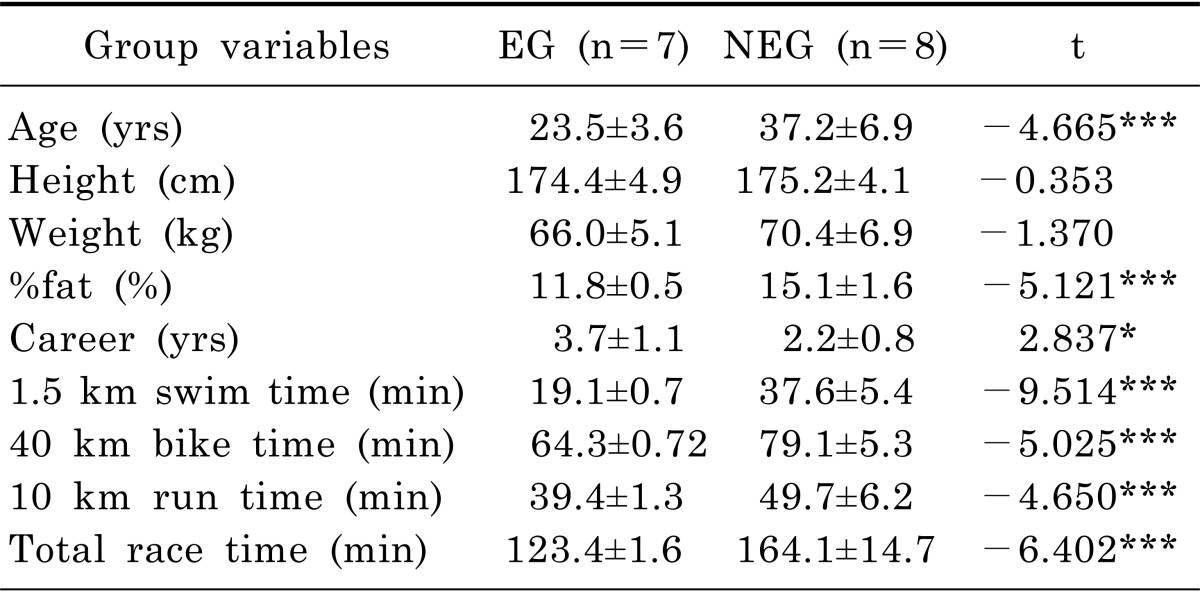
The 15 participants who completed Olympic triathlon were classified as well-trained national team triathletes (elite group [EG], n=7) or amateur non-elite triathletes (non-elite group [NEG], n=8). None of the participants suffered from any acute or chronic disease, and reported medication intake, including antioxidants and nicotine abuse. The subjects are shown in Table 1. All participants agreed and signed an informed consent approved by the Institutional Human Research Ethics Committee. We have read and understood the ethical standards document and confirmed that our study meets the ethical standards (DIRB-201309-HR-R-005) [19].
Subjects were permitted to drink and eat ad libitum during their performance and no particular conduct was given to participants.
Blood was taken from an antecubital vein. Three days before the race, baseline blood samples were collected after an overnight fasting (8 hours). Post race blood samples were collected in the supine position immediately, 2 hours, and 7 days following the triathlon.
LDH and total CK were evaluated by the Modular System P (Roche Diagnostics GmbH, Germany). Cardiac troponin T (cTnT), CK-MB and myoglobin were assayed by the Elecsys 1010 (Roche). Although the detection limit for cTnT is 0.01 ng/mL, the recommended diagnostic threshold for myocardial damage is >0.03 ng/mL. Hemoglobin and hematocrit were measured on ADVIA 120 (Bayer Diagnostics, Newbury, UK).
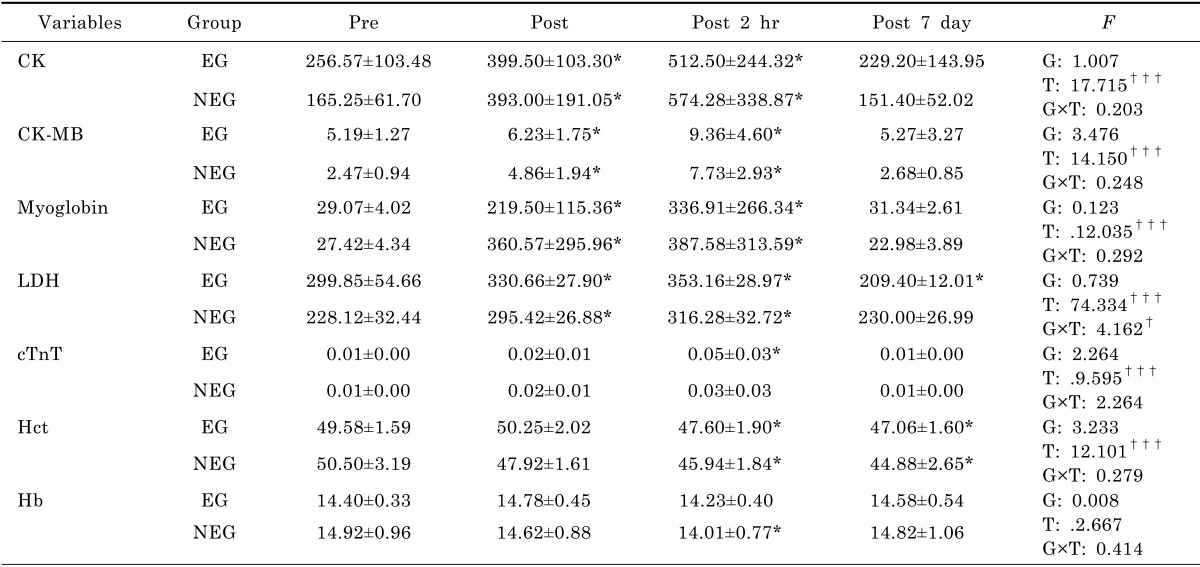
All the athletes completed Olympic triathlon event successfully. Table 2 shows biomarkers of myocardial damage before, immediately, 2 hours and 7 days after finishing the race.
There were no significant differences between the groups in any variable at baseline. CK-MB, CK, myoglobin, and LDH were significantly increased in both groups immediately and 2 hours after the event (p<.05). CK, CK-MB, and myoglobin were completely recovered after the post 7 days (p>.05), whereas, Hct was significantly decreased 7 days after the performance in both groups (p<.05). LDH was significantly decreased 7 days after the performance in the EG (p<.05).
The cTnT was unchanged immediately after the triathlon race in both groups, but it was significantly increased 2 hours after the race in the EG (p<.05), but not in the NEG group. cTnT was also completely recovered 7 days after the race in both groups.
Hct was unchanged immediately after the triathlon race in both groups, but it was significantly decreased in both groups at 2 hours and 7 days after the race (p<.05). Hemoglobin was unchanged immediately after the triathlon race in both groups (p<.05), but it significantly decreased at 2 hours after the race in the NEG (p<.05), but not in the EG. Hemoglobin was recovered 7 days after the race in both groups.
Cardiac injury after strenuous physical exercise in healthy individuals is relatively well known, however the effect of Olympic triathlon on cardiac damage in elite and non-elite athletes has not been evaluated. In the present study, we found that cardiac damage occurs during a triathlon race and elite triathletes had more improvement than the non-elite triathletes. However, all cardiac damage markers return to normal range within 1 week.
Young athletes are widely regarded as a unique subgroup of healthy individuals. However, sudden death of young athletes was regarded as a mysterious and undefined syndrome [20]. Transient cardiac ventricular dysfunction or sudden cardiac death has been reported in male athletes participating in marathon racing [1,2,21,22]. Vigorous exercise is associated with a transient increase in risk of sudden cardiac death. For young athletes (12 to 35 years) participating in competitive sports, the total relative risk of sudden cardiac death is approximately 2.5 times higher than in nonathletes [22]. The incidence of sudden cardiac death in older athletes (>35 years old) is reportedly more frequent than in the young, with an estimated annual incidence of approximately 1:11,000 to 1:80,000 in men [21]. In cTnT change after marathon race, 55 (11%) among 482 participant had significant increases and young age (<30 years) were associated with elevated troponins [2].
Myocardial injury after strenuous exercise in healthy individuals is well indicated and may be the result of exercise-induced production of free radicals [23]. Muscle soreness results from skeletal muscle damage and prolonged and intense training induces a subsequent release of biochemical indexes, including CK-MB, CK, LDH and myoglobin [24,25]. One possible explanation for endurance performance is that less muscle activation would be needed to perform a task when a muscle is stronger, hence delaying fatigue [26]. In response to exercise, skeletal muscle is a highly adaptable tissue and capable of regeneration in response to injury. The adaptation of skeletal muscles is governed by three major processes: satellite cell activity, gene transcription, and protein translation. Exercise parameters such as intensity, duration or average energy expenditure increase typical markers of skeletal muscle damage. Previous study indicated that the post-training elevations of CK values are higher in elite athletes [27]. However, the significant increase in CK levels are usually found in non-trained than in trained subjects [28].
Myoglobin, in fact, is released from the injured tissue more rapidly, which explains an earlier peak in plasma and faster return to baseline levels than CK-MB, CK and LDH. In the present study, CK and myoglobin increased in both groups immediately and 2 hours after the race. We found that the transient increase in CK was higher in NEG than in the EG. However, despite the great severity of the triathlon, these results are comparable to the results observed in marathon runners [15]. This suggests that the release of these biochemical markers into the circulation may be stabilized in ultra-endurance events [18,29]. In previous studies CK and myoglobin have decreased after five days post-race in the Ironman triathlon, but were still significantly elevated compared to the levels observed immediately after the event [4]. However, in the present study revealed that CK, CK-MB, myoglobin and LDH level were completely recovered 7 days after the race.
The time of CK release into and clearance from plasma depends on the type, duration and level of training [29]. The peak levels of serum CK, which are about 2-fold above baseline, occurs 8 hours after exhaustive acute exercise. Increased CK levels after muscle damage induced by eccentric exercise are elevated between 2 and 7 days after exercise. Previous study indicated that CK-MB is a more sensitive indicator induced by myocardial damage than the level of CK itself activity, because it has a lower level and has a much narrow range [29].
It is known that CK-MB values are elevated during 4 to 6 hours, peak at 10 to 24 hours, and return to normal levels within 3 to 4 days after an acute myocardial infarction [25]. We found that CK-MB concentrations were also significantly increased in both groups immediately and 2 hours after the race. Although CK-MB is a more specific marker of myocardial necrosis, its specificity decreases in the presence of significant skeletal muscle damage. Therefore, CK-MB cannot be determined to the specific indicator of myocardial damage induced in athletes following olympic triathlon [25].
A reduction in left ventricular systolic and diastolic function subsequent to prolonged exercise in healthy humans, often called exercise-induced cardiac fatigue (EICF), has been reported in the literature [7,13,39]. If myocardial damage occurs with prolonged exercise such as marathon, cycling and triathlon, this would result in the release of myocardial cellular proteins into the general circulation, where they can be detected.
Several previous studies have indicated that the release of cardiac troponins as they are considered to be more specific markers of myocardial damage occurs after prolonged and strenuous endurance exercise [4,6,7,11,15,17,18]. However, there are some opposite results about the appearance of cardiac troponins. There is some evidence that intensive swimming for 5 hours could cause necrosis of myocardial cells [31]. Several other studies have reported no evidence of cardiac muscle damage after prolonged endurance exercise [31,32]. Although there is exercise-induced skeletal muscle damage shown by increased CK and CK-MB, cTnT was not increased and left ventricular function was unaffected [33]. In contrast, Leetmaa et al. reported that cTnT increased immediately, but decreased to normal limits within 12-24 hours post-triathlon race [34]. In the current study, we found that the cTnT remained unchanged immediately after the triathlon race in both groups, but it was significantly increased 2 hours after the race in the elite group. It appears that the high exercise intensity explains in part the myocardial injury, as race time is inversely associated with cTnT after a triathlon [35]. Troponin is a contractile protein and comprises 5 percent of muscle proteins. It has a key role in the regulation of the calcium mediated muscle contraction through an interaction with actin and myosin [36]. After myocardial damage, troponins released from cardiomyocytes are detectable in peripheral blood within 3~10 hours [27]. Myocardial damage could be induced by various factors, including coronary artery spasms, wall stress, increased stress on atherosclerotic plaques, ischemia, and strenuous exercise. The mechanism of cTnT release after exhaustive exercise and myocardial infarction may somewhat be different. A possible role of catecholamine-induced myocardial damage after triathlon has been proposed [33].
The physiologic stress imposed by the triathlon race resulted in increased CK, CK-MB, myoglobin and LDH levels in elite and non-elite male triathletes. CK, CK-MB, myoglobin and LDH are associated with exercise-induced skeletal muscle damage and cTnT may be used to find myocardial damage following triathlon race. But the mechanisms behind such altered cardiac damage after triathlon race are yet to be found.
In this study, although there is exercise-induced skeletal muscle damage shown by increased CK and CK-MB, cTnT was not increased in both groups immediately after the race. But there was the significant increase in cTnT after 2 hours in EG. We can suggest that it was due to the enormous exercise stress for high performance imposed in EG.
However, the main limitation of the present study is that we cannot absolutely sure that without the investigation of left ventricular function during triathlon. The underlying mechanisms responsible for the myocardial damage observed in the present study remain to be fully elucidated and warrant further investigation.
There are many mechanisms that contribute to an increased tissue oxygen supply during exercise. Generally, it was well known that training increase total Hb mass by stimulating erythropoiesis. The main function of erythrocytes is to transport the oxygen carrier, Hb. For the most part, Hb and Hct are associated with sports anemia. Previous study had reported that Hb and Hct values were increased immediately after Ironman triathlon, but these responses did not occur immediately after Olympic triathlon [37]. Hct increase during exercise due to a decrease in plasma volume when fluid replacement during exercise is insufficient [37]. In this study, Hb was unchanged immediately after the race in both groups, but it decreased at 2 hours after the race in the NEG. The reason for the different result of Hb between both groups at 2 hours after the race is that Hb was influenced by training status, which is probably one important reason for their high performance in endurance athletes.
ACKNOWLEDGEMENTS
This research was supported by Dong-Eui University Research Fund, 2014 (2014AA099) and was also supported by National Research Foundation of Korea Grant funded by the Korean Government [NRF-2012-2012S1A5B5A070-35980].
References
1. Frassl W, Kowoll R, Katz N, Speth M, Stangl A, Brechtel L, Joscht B, Boldt LH, Meier-Buttermilch R, Schlemmer M, Roecker L, Gunga HC. Cardiac markers (BNP, NT-pro-BNP, Troponin I, Troponin T, in female amateur runners before and up until three days after a marathon. Clin Lab. 2008; 54:81–87. PMID: 18630737.
2. Fortescue EB, Shin AY, Greenes DS, Mannix RC, Agarwal S, Feldman BJ, Shah MI, Rifai N, Landzberg MJ, Newburger JW, Almond CS. Cardiac troponin increases among runners in the Boston Marathon. Ann Emerg Med. 2007; 49:137–143. PMID: 17145114.

3. Kratz A, Lewandrowski KB, Siegel AJ, Chun KY, Flood JG, Van Cott EM, Lee-Lewandrowski E. Effect of marathon running on hematologic and biochemical laboratory parameters, including cardiac markers. Am J Clin Pathol. 2002; 118:856–863. PMID: 12472278.

4. Neubauer O, König D, Wagner KH. Recovery after an Ironman triathlon: sustained inflammatory responses and muscular stress. Eur J Appl Physiol. 2008; 104:417–426. PMID: 18548269.

5. Sulzer NU, Schwellnus MP, Noakes TD. Serum electrolytes in Ironman triathletes with exercise-associated muscle cramping. Med Sci Sports Exerc. 2005; 37:1081–1085. PMID: 16015122.

6. Suzuki K, Peake J, Nosaka K, Okutsu M, Abbiss CR, Surriano R, Bishop D, Quod MJ, Lee H, Martin DT, Laursen PB. Changes in markers of muscle damage, inflammation and HSP70 after an Ironman Triathlon race. Eur J Appl Physiol. 2006; 98:525–534. PMID: 17031693.

7. Tulloh L, Robinson D, Patel A, Ware A, Prendergast C, Sullivan D, Pressley L. Raised troponin T and echocardiographic abnormalities after prolonged strenuous exercise--the Australian Ironman Triathlon. Br J Sports Med. 2006; 40:605–609. PMID: 16611724.

8. Kim K, Kyung T, Kim W, Shin C, Song Y, Lee MY, Lee H, Cho Y. Efficient management design for swimming exercise treatment. Korean J Physiol Pharmacol. 2009; 13:497–502. PMID: 20054498.

9. O'Toole ML, Hiller WD, Roalstad MS, Douglas PS. Hemolysis during triathlon races: its relation to race distance. Med Sci Sports Exerc. 1988; 20:272–275. PMID: 3386507.
10. Park CH, Park TG, Kim TU, Kwak YS. Changes of immunlogical markers in elite and amateur triathletes. ISMJ. 2008; 9:116–130.
11. Lippi G, Schena F, Salvagno GL, Montagnana M, Gelati M, Tarperi C, Banfi G, Guidi GC. Acute variation of biochemical markers of muscle damage following a 21-km, half-marathon run. Scand J Clin Lab Invest. 2008; 68:667–672. PMID: 18609111.

12. Park CH, Kwak YS, Kim TU. Triathlon-related overuse injury and medical issues. J Life Sci. 2010; 20:314–320.

13. Whyte GP. Clinical significance of cardiac damage and changes in function after exercise. Med Sci Sports Exerc. 2008; 40:1416–1423. PMID: 18614951.

14. Lim CL, Mackinnon LT. The roles of exercise-induced immune system disturbances in the pathology of heat stroke : the dual pathway model of heat stroke. Sports Med. 2006; 36:39–64. PMID: 16445310.
15. Smith JE, Garbutt G, Lopes P, Pedoe DT. Effects of prolonged strenuous exercise (marathon running) on biochemical and haematological markers used in the investigation of patients in the emergency department. Br J Sports Med. 2004; 38:292–294. PMID: 15155430.

16. Engel G, Rockson SG. Rapid diagnosis of myocardial injury with troponin T and CK-MB relative index. Mol Diagn Ther. 2007; 11:109–116. PMID: 17397247.

17. Brancaccio P, Maffulli N, Limongelli FM. Creatine kinase monitoring in sport medicine. Br Med Bull. 2007; 81-82:209–230. PMID: 17569697.

18. Jassal DS, Moffat D, Krahn J, Ahmadie R, Fang T, Eschun G, Sharma S. Cardiac injury markers in non-elite marathon runners. Int J Sports Med. 2009; 30:75–79. PMID: 19177312.

19. Harriss DJ, Atkinson G. Ethical standards in sport and exercise science research. Int J Sports Med. 2009; 30:701–702. PMID: 19809942.
20. Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation. 2006; 114:1633–1644. PMID: 17030703.
21. Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000; 343:1355–1361. PMID: 11070099.

22. Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003; 42:1959–1963. PMID: 14662259.

23. Kanter MM. Free radicals, exercise, and antioxidant supplementation. Int J Sport Nutr. 1994; 4:205–220. PMID: 7987357.

24. Farber H, Arbetter J, Schaeffer E, Hill S, Dallal G, Grimaldi R, Hill N. Acute metabolic effects of an endurance triathlon. Ann Sports Med. 1987; 3:131–138.
25. Roth HJ, Leithäuser RM, Doppelmayr H, Doppelmayr M, Finkernagel H, von Duvillard SP, Korff S, Katus HA, Giannitsis E, Beneke R. Cardiospecificity of the 3rd generation cardiac troponin T assay during and after a 216 km ultra-endurance marathon run in Death Valley. Clin Res Cardiol. 2007; 96:359–364. PMID: 17453141.

26. Frontera WR, Meredith CN, O'Reilly KP, Evans WJ. Strength training and determinants of VO2max in older men. J Appl Physiol (1985). 1990; 68:329–333. PMID: 2312474.

27. Mair J, Thome-Kromer B, Wagner I, Lechleitner P, Dienstl F, Puschendorf B, Michel G. Concentration time courses of troponin and myosin subunits after acute myocardial infarction. Coron Artery Dis. 1994; 5:865–872. PMID: 7866607.
28. Fehrenbach E, Niess AM, Schlotz E, Passek F, Dickhuth HH, Northoff H. Transcriptional and translational regulation of heat shock proteins in leukocytes of endurance runners. J Appl Physiol (1985). 2000; 89:704–710. PMID: 10926657.

29. Yamin C, Oliveira J, Meckel Y, Eynon N, Sagiv M, Ayalon M, Alves AJ, Duarte JA. CK-MM gene polymorphism does not influence the blood CK activity levels after exhaustive eccentric exercise. Int J Sports Med. 2010; 31:213–217. PMID: 20157874.

30. Douglas PS, O'Toole ML, Katz SE. Prolonged exercise alters cardiac chronotropic responsiveness in endurance athletes. J Sports Med Phys Fitness. 1998; 38:158–163. PMID: 9763802.
31. Chen Y, Serfass RC, Mackey-Bojack SM, Kelly KL, Titus JL, Apple FS. Cardiac troponin T alterations in myocardium and serum of rats after stressful, prolonged intense exercise. J Appl Physiol (1985). 2000; 88:1749–1755. PMID: 10797139.

32. König D, Schumacher YO, Heinrich L, Schmid A, Berg A, Dickhuth HH. Myocardial stress after competitive exercise in professional road cyclists. Med Sci Sports Exerc. 2003; 35:1679–1683. PMID: 14523304.
33. Shave R, Dawson E, Whyte G, George K, Ball D, Collinson P, Gaze D. The cardiospecificity of the third-generation cTnT assay after exercise-induced muscle damage. Med Sci Sports Exerc. 2002; 34:651–654. PMID: 11932574.

34. Leetmaa TH, Dam A, Glintborg D, Markenvard JD. Myocardial response to a triathlon in male athletes evaluated by Doppler tissue imaging and biochemical parameters. Scand J Med Sci Sports. 2008; 18:698–705. PMID: 18248532.

35. Michielsen EC, Wodzig WK, Van Dieijen-Visser MP. Cardiac troponin T release after prolonged strenuous exercise. Sports Med. 2008; 38:425–435. PMID: 18416595.

36. Mair J, Dienstl F, Puschendorf B. Cardiac troponin T in the diagnosis of myocardial injury. Crit Rev Clin Lab Sci. 1992; 29:31–57. PMID: 1388708.

37. Park CH, Kim TU. The effect of different triathlon on weight, sodium and hematological changes. J Life Sci. 2009; 19:46–51.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download