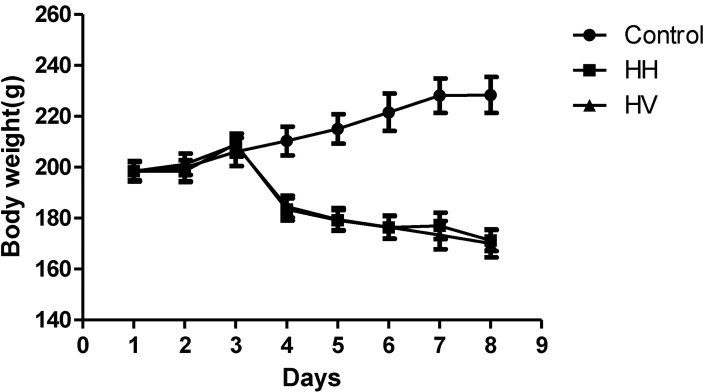
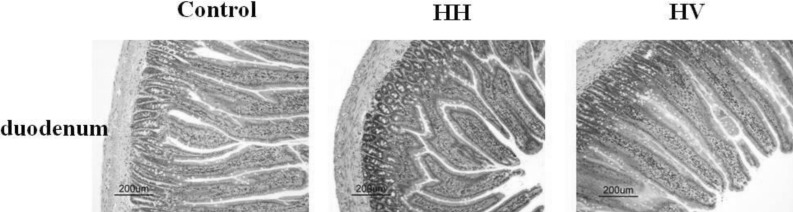
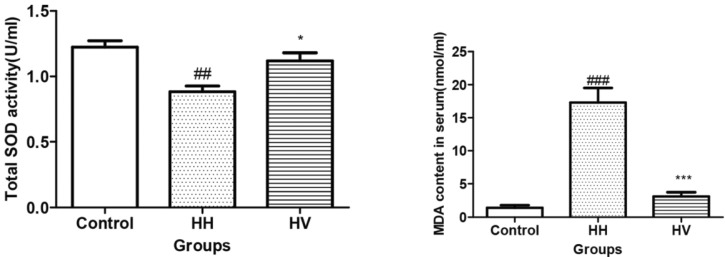
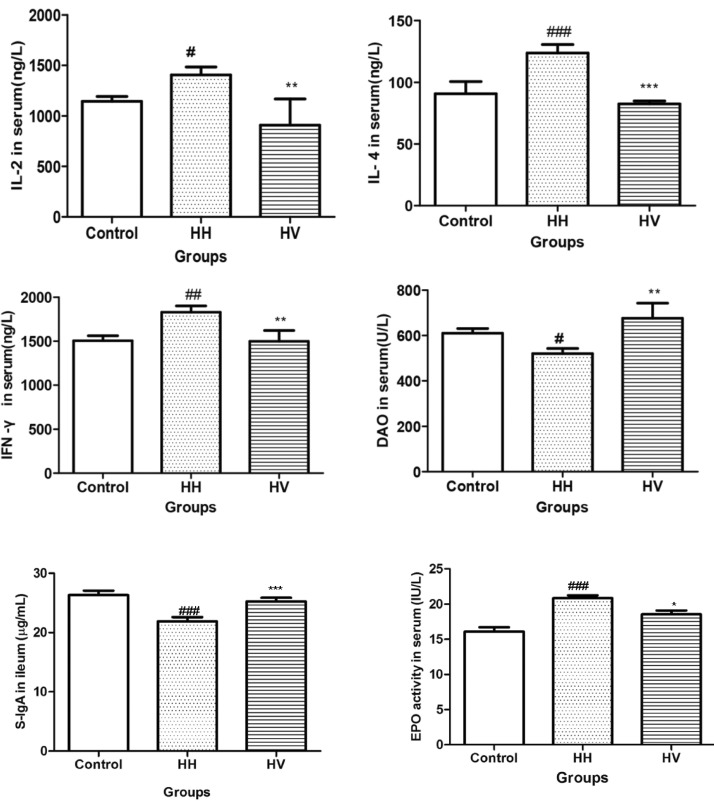
1. Zhou B, Yang DZ, Zhou QQ. The SEM observation of small intestinal mucosa in the rabbits under simulated high altitude hypoxia. Chin J Gastroenterol Hepatol. 2009; 18:751–753.
2. Recavarren-Arce S, Ramirez-Ramos A, Gilman RH, Chinga-Alayo E, Watanabe-Yamamoto J, Rodriguez-Ulloa C, Miyagui J, Passaro DJ, Eza D. Severe gastritis in the Peruvian Andes. Histopathology. 2005; 46:374–379. PMID:
15810948.

3. Shen L. Functional morphology of the gastrointestinal tract. Curr Top Microbiol Immunol. 2009; 337:1–35. PMID:
19812978.

4. Zhou QQ, Yang DZ, Luo YJ, Li SZ, Liu FY, Wang GS. Over-starvation aggravates intestinal injury and promotes bacterial and endotoxin translocation under high-altitude hypoxic environment. World J Gastroenterol. 2011; 17:1584–1593. PMID:
21472125.

5. Wu WM, Zhang FX. Research advances in plateau hypoxia and gut barrier injury. World Chinese J Digestol. 2009; 17:1432–1436.

6. Tang XL, Xu MJ, Li ZH, Pan Q, Fu JH. Effects of vitamin E on expressions of eight microRNAs in the liver of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2013; 34:1470–1475. PMID:
23542605.

7. Ernst IM, Pallauf K, Bendall JK, Paulsen L, Nikolai S, Huebbe P, Roeder T, Rimbach G. Vitamin E supplementation and lifespan in model organisms. Ageing Res Rev. 2013; 12:365–375. PMID:
23099151.

8. Finno CJ, Valberg SJ. A comparative review of vitamin E and associated equine disorders. J Vet Intern Med. 2012; 26:1251–1266. PMID:
22925200.

9. Vavricka SR, Rogler G. Intestinal absorption and vitamin levels: is a new focus needed? Dig Dis. 2012; 30(Suppl 3):73–80. PMID:
23295695.

10. Bailey DM, Davies B. Acute mountain sickness; prophylactic benefits of antioxidant vitamin supplementation at high altitude. High Alt Med Biol. 2001; 2:21–29. PMID:
11252695.

11. Ilavazhagan G, Bansal A, Prasad D, Thomas P, Sharma SK, Kain AK, Kumar D, Selvamurthy W. Effect of vitamin E supplementation on hypoxia-induced oxidative damage in male albino rats. Aviat Space Environ Med. 2001; 72:899–903. PMID:
11601553.
12. Lee JD, Choi HC, Kang YJ, Kim MS, Lee KY. Effects of Antioxidants on the gamma-radiation damage of the cultured vascular smooth mucle cells of rat aorta. Korean J Physiol Pharmacol. 2007; 11:189–195.
13. Luo H, Guo P, Zhou Q. Role of TLR4/NF-κB in damage to intestinal mucosa barrier function and bacterial translocation in rats exposed to hypoxia. PLoS One. 2012; 7:e46291. PMID:
23082119.

14. Schulz O, Pabst O. Antigen sampling in the small intestine. Trends Immunol. 2013; 34:155–161. PMID:
23083727.

15. Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013; 167:374–379. PMID:
23400224.

16. Rist VT, Weiss E, Eklund M, Mosenthin R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: a review. Animal. 2013; 7:1067–1078. PMID:
23410993.

17. Roche M, Kemp FW, Agrawal A, Attanasio A, Neti PV, Howell RW, Ferraris RP. Marked changes in endogenous antioxidant expression precede vitamin A-, C-, and E-protectable, radiation-induced reductions in small intestinal nutrient transport. Free Radic Biol Med. 2011; 50:55–65. PMID:
20970494.

18. Mena S, Ortega A, Estrela JM. Oxidative stress in environmental-induced carcinogenesis. Mutat Res. 2009; 674:36–44. PMID:
18977455.

19. Seth V, Banerjee BD, Chakravorty AK. Lipid peroxidation, free radical scavenging enzymes, and glutathione redox system in blood of rats exposed to propoxur. Pestic Biochem Physiol. 2001; 71:133–139.

20. Uzun FG, Demir F, Kalender S, Bas H, Kalender Y. Protective effect of catechin and quercetin on chlorpyrifos-induced lung toxicity in male rats. Food Chem Toxicol. 2010; 48:1714–1720. PMID:
20381572.

21. Najeed Q, Bhaskar N, Masood I, Wadhwa S, Kaur H, Ishaq S. Malondialdehyde (MDA) and superoxide dismutase (SOD) levels-distinguishing parameters between benign and malignant pleural effusions. Free Radic Antioxid. 2012; 2:8–11.
22. Singh M, Thomas P, Shukla D, Tulsawani R, Saxena S, Bansal A. Effect of subchronic hypobaric hypoxia on oxidative stress in rat heart. Appl Biochem Biotechnol. 2013; 169:2405–2419. PMID:
23456277.

23. El-Demerdash FM, Jebur AB, Nasr HM. Oxidative stress and biochemical perturbations induced by insecticides mixture in rat testes. J Environ Sci Health B. 2013; 48:593–599. PMID:
23581693.

24. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007; 448:427–434. PMID:
17653185.

25. Choi WJ, Kim SK, Park HK, Sohn UD, Kim W. Anti-Inflammatory and Anti-Superbacterial Properties of Sulforaphane from Shepherd's Purse. Korean J Physiol Pharmacol. 2014; 18:33–39. PMID:
24634594.

26. Wu QJ, Zhou YM, Wu YN, Zhang LL, Wang T. The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens. Vet Immunol Immunopathol. 2013; 153:70–76. PMID:
23453767.

27. Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011; 23:598–604. PMID:
21889323.

28. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004; 75:163–189. PMID:
14525967.
29. Hansen CH, Frøkiær H, Christensen AG, Bergström A, Licht TR, Hansen AK, Metzdorff SB. Dietary xylooligosaccharide downregulates IFN-γ and the low-grade inflammatory cytokine IL-1β systemically in mice. J Nutr. 2013; 143:533–540. PMID:
23427328.

30. Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007; 132:1359–1374. PMID:
17408640.

31. Muir WI, Husband AJ, Bryden WL. Dietary supplementation with vitamin E modulates avian intestinal immunity. Br J Nutr. 2002; 87:579–585. PMID:
12067428.

32. Elmore BO, Bollinger JA, Dooley DM. Human kidney diamine oxidase: heterologous expression, purification, and characterization. J Biol Inorg Chem. 2002; 7:565–579. PMID:
12072962.

33. Hamada Y, Shinohara Y, Yano M, Yamamoto M, Yoshio M, Satake K, Toda A, Hirai M, Usami M. Effect of the menstrual cycle on serum diamine oxidase levels in healthy women. Clin Biochem. 2013; 46:99–102. PMID:
23099198.

34. Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009; 70:505–515. PMID:
19906191.

35. Shang X, Wang P, Liu Y, Zhang Z, Xue Y. Mechanism of low-frequency ultrasound in opening blood-tumor barrier by tight junction. J Mol Neurosci. 2011; 43:364–369. PMID:
20852968.

36. Rao R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann N Y Acad Sci. 2009; 1165:62–68. PMID:
19538289.

37. Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005; 288:C1231–C1241. PMID:
15689410.

38. Basu M, Malhotra AS, Pal K, Prasad R, Kumar R, Prasad BA, Sawhney RC. Erythropoietin levels in lowlanders and high-altitude natives at 3450 m. Aviat Space Environ Med. 2007; 78:963–967. PMID:
17955945.

39. Höpfl G, Ogunshola O, Gassmann M. Hypoxia and high altitude. The molecular response. Adv Exp Med Biol. 2003; 543:89–115. PMID:
14713116.
40. Zamudio S, Wu Y, Ietta F, Rolfo A, Cross A, Wheeler T, Post M, Illsley NP, Caniggia I. Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J Pathol. 2007; 170:2171–2179. PMID:
17525282.
41. Fedele AO, Whitelaw ML, Peet DJ. Regulation of gene expression by the hypoxia-inducible factors. Mol Interv. 2002; 2:229–243. PMID:
14993394.

42. Chepelev NL, Willmore WG. Regulation of iron pathways in response to hypoxia. Free Radic Biol Med. 2011; 50:645–666. PMID:
21185934.

43. Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest. 2007; 117:1926–1932. PMID:
17557118.

44. Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999; 15:551–578. PMID:
10611972.

45. Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, Tatevian N, Rhoads JM. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2009; 297:G442–G450. PMID:
19608731.

46. Weber-Mzell D, Zaupa P, Petnehazy T, Kobayashi H, Schimpl G, Feierl G, Kotanko P, Hollwarth M. The role of nuclear factor-kappa B in bacterial translocation in cholestatic rats. Pediatr Surg Int. 2006; 22:43–49. PMID:
16333628.