ABBREVIATIONS
AM
AMPA
ATP
DMEM
EGTA
ER
FBS
H2DCFDA
HEPES-HBSS
HS
IP3
2-MeSATP
NMDA
PC12
PKC
PLC
ROS
SOC
SOCE
INTRODUCTION
METHODS
Materials
Preparation of cyanidin-3-glucoside
Cell culture
Digital calcium imaging
Measurement of mitochondrial membrane potential
Measurement of ROS production
Statistical analyses
RESULTS
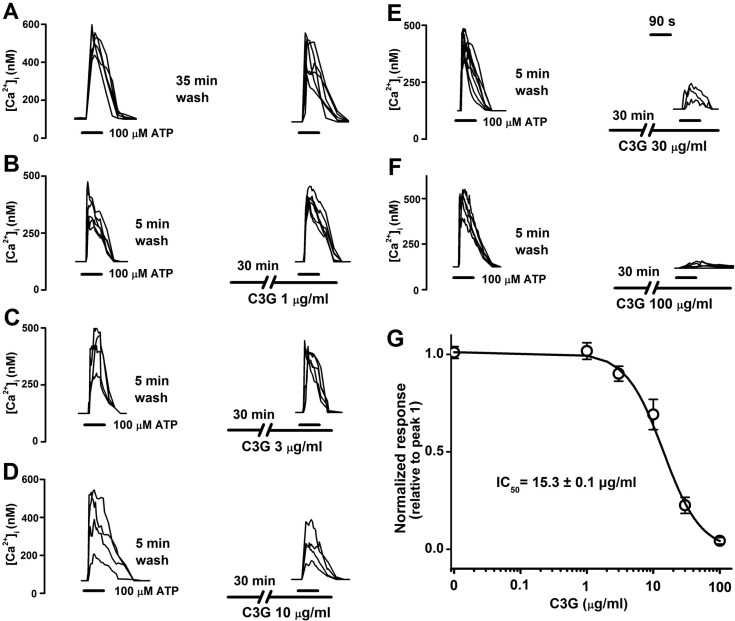 | Fig. 1Concentration-dependent inhibitory effects of cyanidin-3-glucoside on ATP-induced [Ca2+]i increase in PC12 cells. (A) Reproducible [Ca2+]i increases were elicited by superfusion with 100 µM ATP for 90 sec at 35 min interval. (B~F) After pretreating cells with various concentration of cyanidin-3-glucoside (1 µg/ml to 100 µg/ml) for 30 min, the subsequent ATP-induced [Ca2+]i responses were observed. ATP and cyanidin-3-glucoside were applied as indicated by the horizontal bars. G, The ATP-induced response is presented as a ratio of the initial control (peak 2/peak 1) (n=28, 23, 25, 23, 26, 11 at 0 µg/ml, 1 µg/ml, 3 µg/ml, 10 µg/ml, 30 µg/ml, 100 µg/ml, respectively). A non-linear least-square fit of the Hill equation to the concentration-response data yielded an IC50 of 15.3±0.1 µg/ml for cyanidin-3-glucoside. Data are means±SEM. |
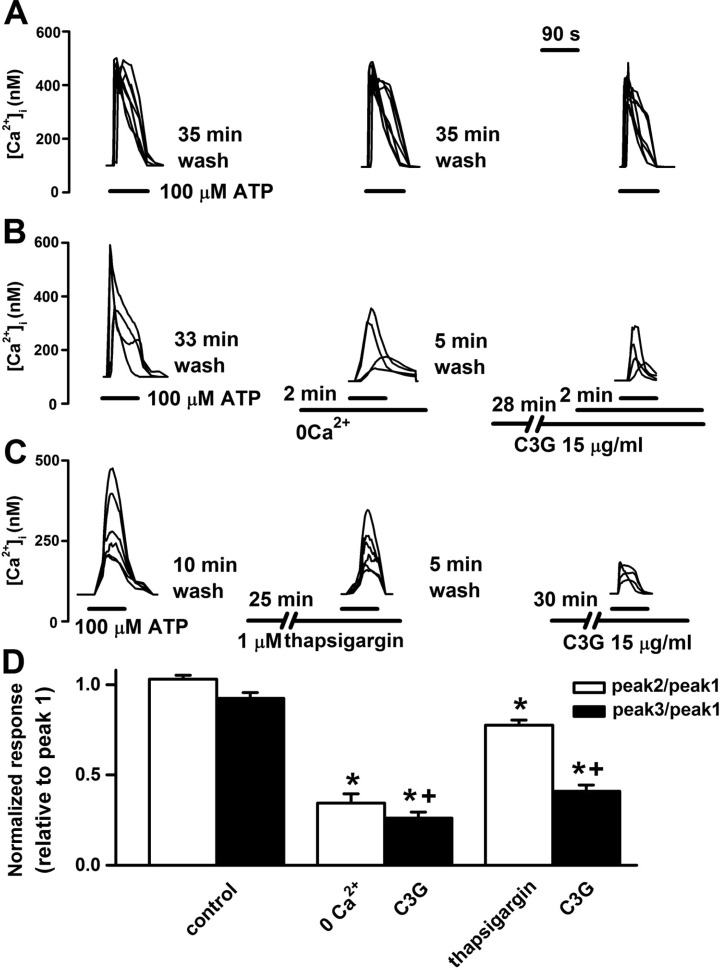 | Fig. 2Inhibitory effects of cyanidin-3-glucoside on ATP-induced a release of Ca2+ from intracellular stores and an influx of Ca2+ from the extracellular space. (A) Reproducible [Ca2+]i increases were elicited by superfusion with 100 µM ATP for 90 sec at 35 min intervals. (B) Removal of extracellular Ca2+ with the 100 µM EGTA-containing Ca2+-free HEPES-HBSS for 2 min inhibited the ATP-induced responses. Pretreatment with cyanidin-3-glucoside (15 µg/ml) for 30 min inhibited the ATP-induced responses in the presence of the Ca2+-free HEPES-HBSS. (C) Pretreatment with thapsigargin (1 µM) for 25 min inhibited the ATP-induced responses. Cyanidin-3-glucoside inhibited the ATP-induced responses after treatment of thapsigargin. (D) The ATP-induced response is presented as a ratio of the initial control (relative to peak 1) after treatment of vehicle (control, n=27), Ca2+-free solution (0 Ca2+, n=22), 0 Ca2+ plus cyanidin-3-glucoside (C3G, n=22), thapsigargin (n=19), and thapsigargin plus cyanidin-3-glucoside (C3G, n=19). Data are means±SEM. *p<0.01 relative to respective control (one-way ANOVA with Bonferroni's test). †p<0.01 relative to 0 Ca2+ or thapsigargin (non-paired Student's t-test). |
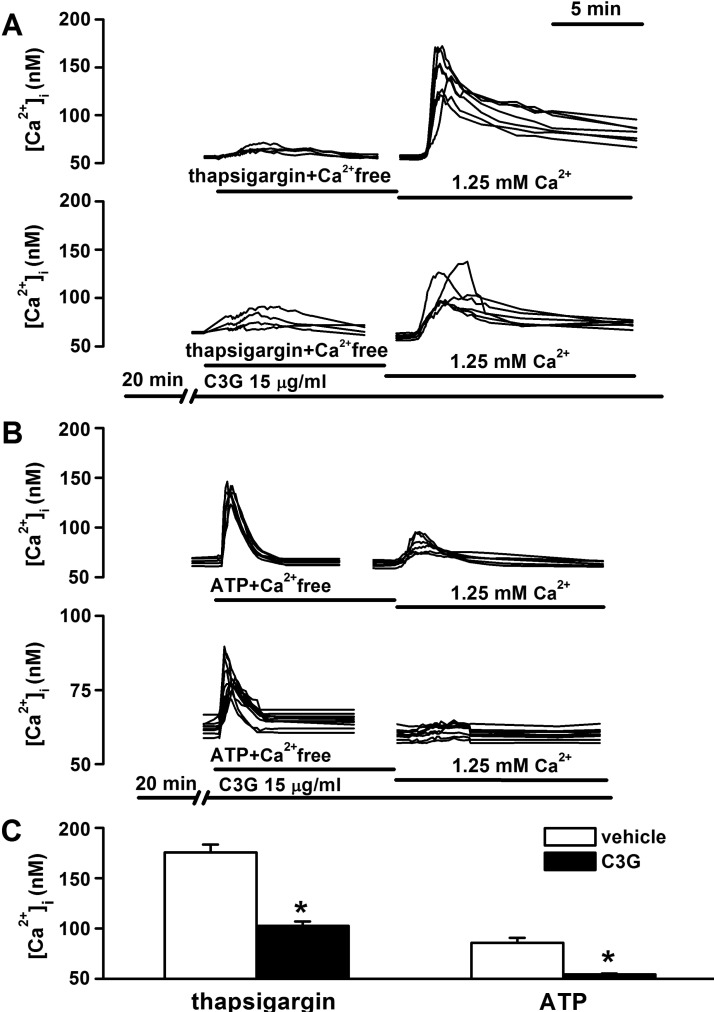 | Fig. 3Inhibitory effects of cyanidin-3-glucoside on thapsigarin or ATP-induced store-operated calcium entry (SOCE). Treatment with thapsigargin (1 µM) (A) or ATP (100 µM) (B)-containing Ca2+-free HEPES-HBSS (100 µM EGTA) for 10 min induced a release of Ca2+ from intracellular stores in the absence or presence of cyanidin-3-glucoside. Subsequent treatment with 1.25 mM Ca2+-containing HEPES-HBSS induced SOCE-induced [Ca2+]i increases. D, The thapsigargin or ATP-induced SOCE is presented as [Ca2+]i increases in non-treated (vehicle, n=27; ATP, n=32) or cyanidin-3-glucoside-treated (vehicle, n=27; ATP, n=33) cells. Data are means± SEM. *p<0.01 relative to respective vehicle (non-paired Student's t-test). |
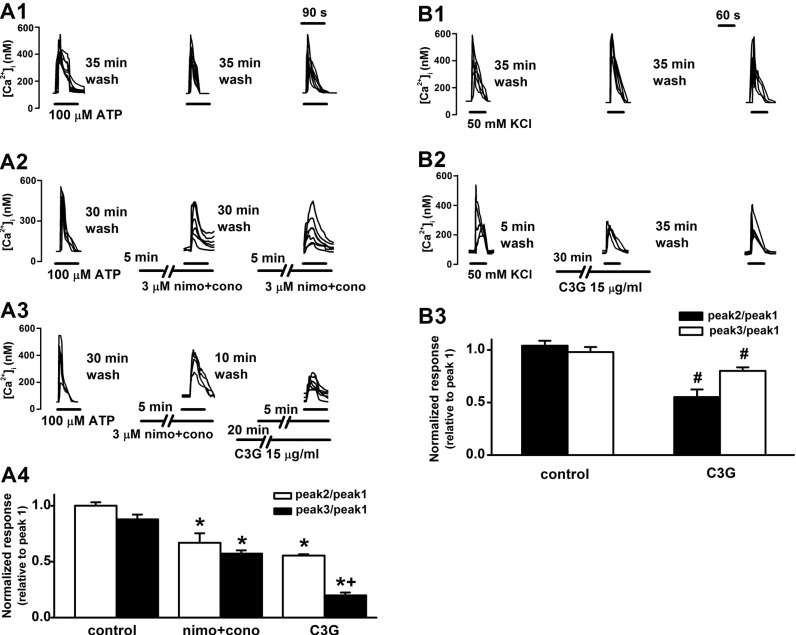 | Fig. 4Inhibitory effects of cyanidin-3-glucoside on ATP or high KCl-induced [Ca2+]i increases through voltage-gated Ca2+ channels. (A) Effects of nimodipine, ω-conotoxin and cyanidin-3-glucoside on ATP (100 µM)-induced [Ca2+]i increases. Reproducible [Ca2+]i increases were elicited by superfusion with 100 µM ATP for 90 sec at 35 min intervals (A1). Pretreatment with both nimodipine (3 µM) and ω-conotoxin (3 µM) for 5 min inhibited the ATP (100 µM)-induced responses (A2). Pretreatment with cyanidin-3-glucoside (15 µg/ml) for 30 min further inhibited the ATP-induced responses in the presence of both nimodipine and ω-conotoxin (A3). The ATP-induced response is presented as a ratio of the initial control (relative to peak 1) after treatment of vehicle (control, n=25), nimodipine and ω-conotoxin (nimo+cono, n=27) and nimodipine and ω-conotoxin plus cyanidin-3-glucoside (C3G, n=23) (A4). (B) Effects of cyanidin-3-glucoside on 50 mM KCl-induced [Ca2+]i increases. Reproducible [Ca2+]i increases were elicited by superfusion with 50 mM KCl-containing HEPES HBSS for 60 sec at 35 min interval (B1). Pretreatment with cyanidin-3-glucoside (15 µg/ml) for 30 min inhibited the high KCl-induced [Ca2+]i responses (B2). The KCl-induced response is presented as a ratio of the initial control after treatment of vehicle (control, n=25) or cyanidin-3-glucoside (C3G, n=26) (B3). Data are means±SEM. *p<0.01 relative to respective control (one-way ANOVA with Bonferroni's test), †p<0.01 relative to nimodipine and ω-conotoxin-treated cells (paired Student's t-test and one-way ANOVA with Bonferroni's test), #p<0.01 relative to respective control (non-paired Student's t-test). |
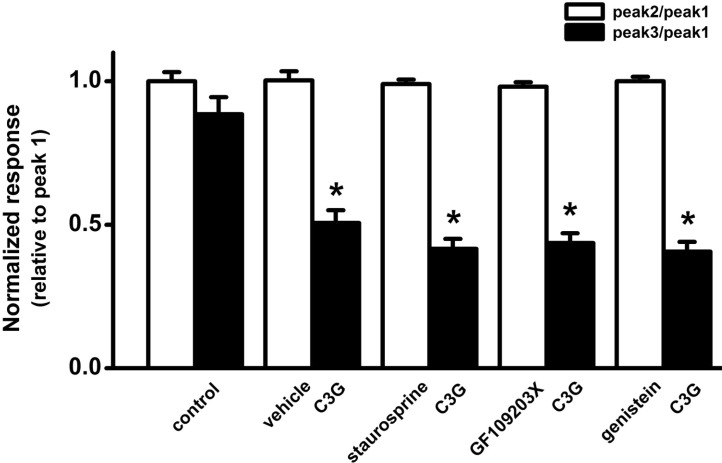 | Fig. 5Effects of protein kinase inhibitors on cyanidin-3-glucoside-induced inhibition of ATP-induced [Ca2+]i increases. A non-specific PKC inhibitor staurosporine, a specific PKC inhibitor GF109203X, and a tyrosine kinase inhibitor genistein did not affect the cyanidin-3-glucoside-induced inhibition of ATP-induced [Ca2+]i increases. The effects of protein kinase inhibitors on cyanidin-3-glucoside-induced inhibition of the ATP-induced responses are presented as a ratio of the initial control (relative to peak 1) after non-treatment (control, n=25) and co-treatment of cyanidin-3-glucoside (15 µg/ml) with vehicle (vehicle, n=21), staurosporine (100 nM, n=29), GF109203X (300 nM, n=31 ), genistein (50 µM, n=32). Data are expressed as means±SEM. *p<0.05 relative to respective control, and respective protein kinase inhibitor (or vehicle) (non-paired Student's t-test). |
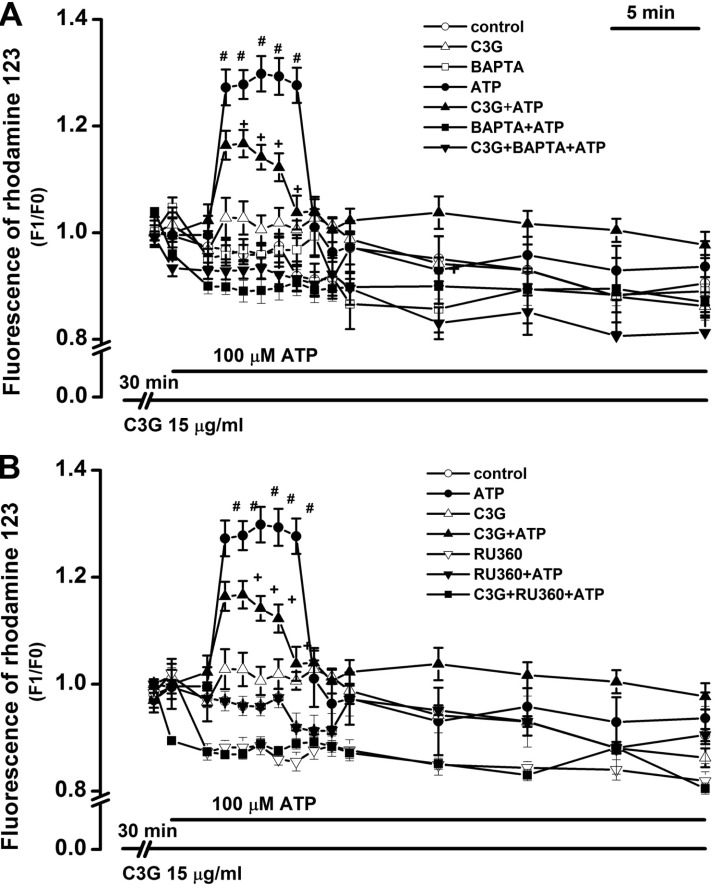 | Fig. 6Inhibitory effects of cyanidin-3-glucoside on ATP-induced depolarization of mitochondrial membrane potential through a Ca2+ influx from the cytosol into the mitochondria. (A) Inhibitory effects of the intracellular Ca2+ chelator BAPTA-AM and cyanidin-3-glucoside on ATP-induced mitochondrial depolarization. Treatment with 100 µM ATP for 30 min induced mitochondrial depolarization (ATP, n=27). Treatment for 30 min with cyanidin-3-glucoside (15 µg/ml) alone did not affect the membrane potential (C3G, n=26), but it significantly inhibited the mitochondrial depolarization (C3G +ATP, n=26). Treatment for 30 min with BAPTA-AM (10 µM) alone did not affect the membrane potential (BAPTA, n=22), but it blocked the ATP-induced mitochondrial depolarization in the absence (BAPTA+ATP, n=22) or presence (C3G+BAPTA+ATP, n=27) of cyanidin-3-glucoside. (B) Inhibitory effects of the mitochondrial Ca2+ uniporter inhibitor RU360 and cyanidin-3-glucoside on ATP-induced mitochondrial depolarization. Treatment for 30 min with RU360 (10 µM) alone did not affect the membrane potential (Ru360, n=23), but it blocked the ATP-induced mitochondrial depolarization in the absence (Ru360+ATP, n=38) or presence (C3G+RU360+ATP, n=35) of cyanidin-3-glucoside. Change in mitochondrial membrane potential was shown as a ratio of the initial intensity of fluorescence (F1/F0). Data are expressed as mean±S.E. #p<0.01 relative to control, C3G, BAPTA, BAPTA+ ATP, C3G+BAPTA+ATP, RU360, RU360+ATP, and C3G+ RU360+ATP (two-way ANOVA with Bonferroni's test). †p<0.05 relative to ATP (two-way ANOVA with Bonferroni's test). |
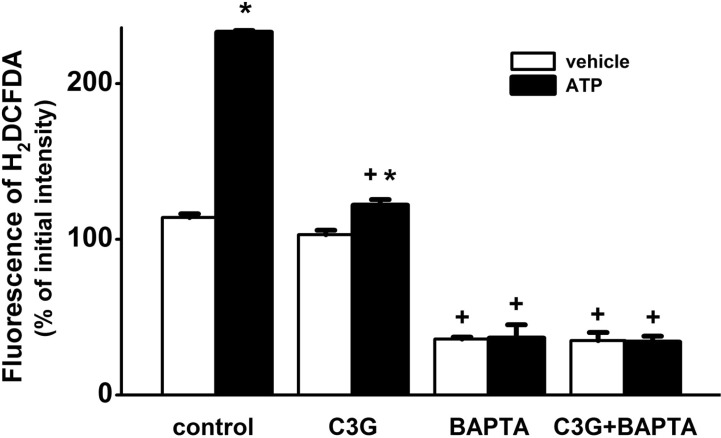 | Fig. 7Inhibitory effects of BAPTA-AM and cyanidin-3-glucoside on ATP-induced formation of ROS. Treatment for 30 min with 100 µM ATP significantly increased formation of ROS. Pretreatment for 30 min with cyanidin-3-glucoside (15 µg/ml) or BAPTA-AM (10 µM) blocked the ATP-induced formation of ROS. The formation of ROS was shown as a percentage of the initial intensity of the fluorescence of H2DCFDA (F1/F0×100) in non-treated, cyanidin-3-glucoside-treated, BAPTA-AM-treated, cyanidin-3-glucoside plus BAPTA-AM-treated in the absence (vehicle, n=24; C3G, n=26; BAPTA, n=22; C3G+BAPTA, n=22, respectively) or presence (vehicle, n=24; C3G, n=26; BAPTA, n=23; C3G+BAPTA, n=23, respectively) of ATP. Data are expressed as mean±S.E. *p<0.01 relative to respective vehicle (non-paired Student's t-test), †p<0.01 relative to respective control and C3G (non-paired Student's t-test). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download