INTRODUCTION
METHODS
Drugs and chemicals
Experimental animals
Experimental procedures
1. Study Design
2. Pentylenetetrazole (PTZ) kindling
Blood sampling, tissue preparation and biochemical measurements
1. Serum lipid profile (mg/dl)
2. Serum total homocysteine (tHcy) measurement
Vascular reactivity studies:
Experimental procedure
Brain studies
1. Hippocampal histopathological studies
2. Hippocampal immunohistochemical staining
Morphometric studies
Aortic and coronaries histopathological studies
Statistical analysis
RESULTS
Effect of lamotrigine on the development of PTZ kindling and seizures scores
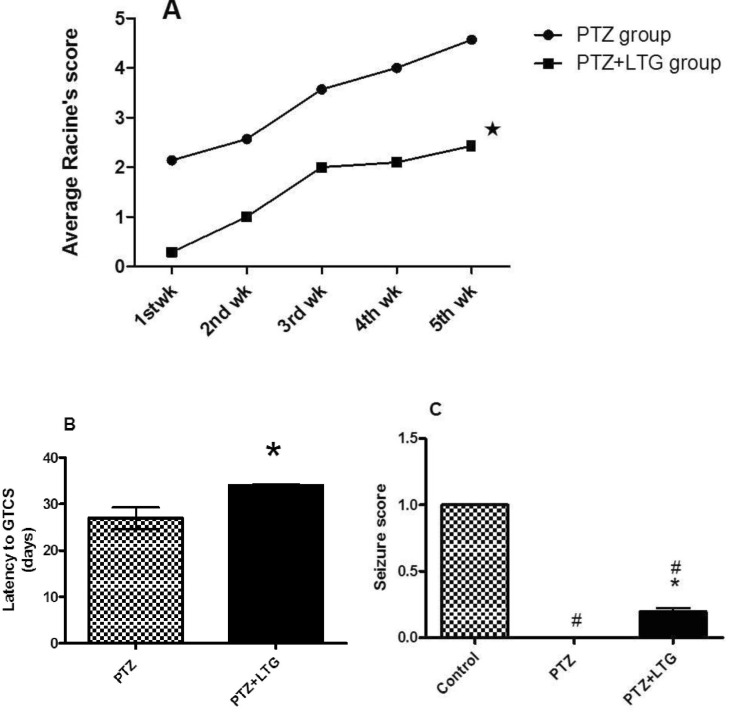 | Fig. 1(A-C) The effect of Pentylenetetrazole and lamotrigine on (A) average Racine score. (B) latency to GTCS (generalized tonic clonic seizures). (C) seizure score. PTZ (Pentylenetetrazole, 30 mg/kg), LTG (lamotrigine, 20 mg/kg). Data are expressed as mean±SD, number of animals=7. For average Racine's score and latency to GTCS, *p<0.05 compared to PTZ were determined by unpaired t-test. For seizure score; #p<0.05 compared to control group, *p<0.05 compared to PTZ group by analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison test. |
Effect of lamotrigine on lipid profile and atherogenic index
Table 1
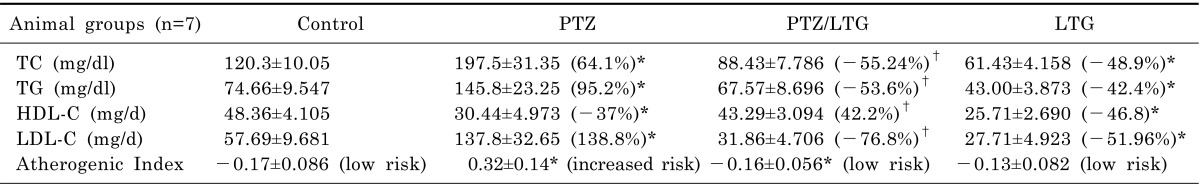
Data are expressed as mean±SD, number of animals (n)=7, PTZ (pentylenetetrazole, 30 mg/kg), LTG (lamotrigine, 20 mg/kg). TC, total cholesterol; TG, triglycerides; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol. *p<0.05 compared to control group, †p<0.05 compared to PTZ group by analysis of variance (ANOVA), followed by Newman-Keuls multiple comparison test.
Effect of lamotrigine on serum total homocysteine level
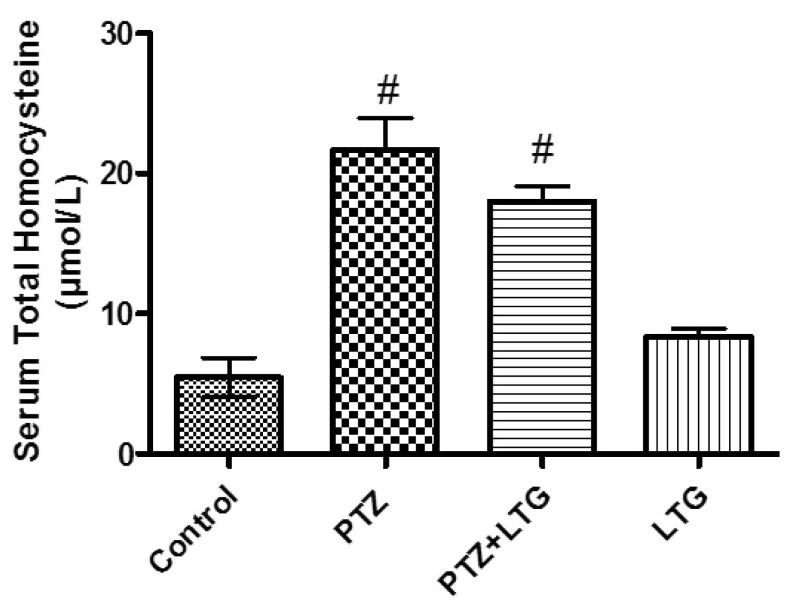 | Fig. 2Serum homocysteine level in control rats, PTZ treated rats, PTZ+LTG and LTG treated rats. PTZ (pentylenetetrazole, 30 mg/kg), LTG (lamotrigine, 20 mg/kg). Data are expressed as mean±SD, number of animals=7. #p<0.05 compared to control group by analysis of variance (ANOVA), followed by Newman-Keuls multiple comparison test. |
Effect of lamotrigine on serum and aortic malondialdehyde (MDA) level
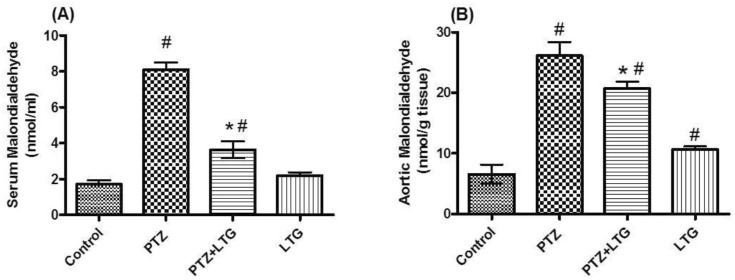 | Fig. 3(A, B) Serum and aortic malondialdehyde levels in control rats, PTZ treated rats, PTZ+LTG and LTG treated rats. PTZ (pentylenetetrazole, 30 mg/kg), LTG (lamotrigine, 20 mg/kg). Data are expressed as mean±SD, number of animals=7. #p<0.05 compared to control group, *p<0.05 compared to PTZ group by analysis of variance (ANOVA), followed by Newman-Keuls multiple comparison test. |
Effect of lamotrigine on serum and aortic reduced glutathione level
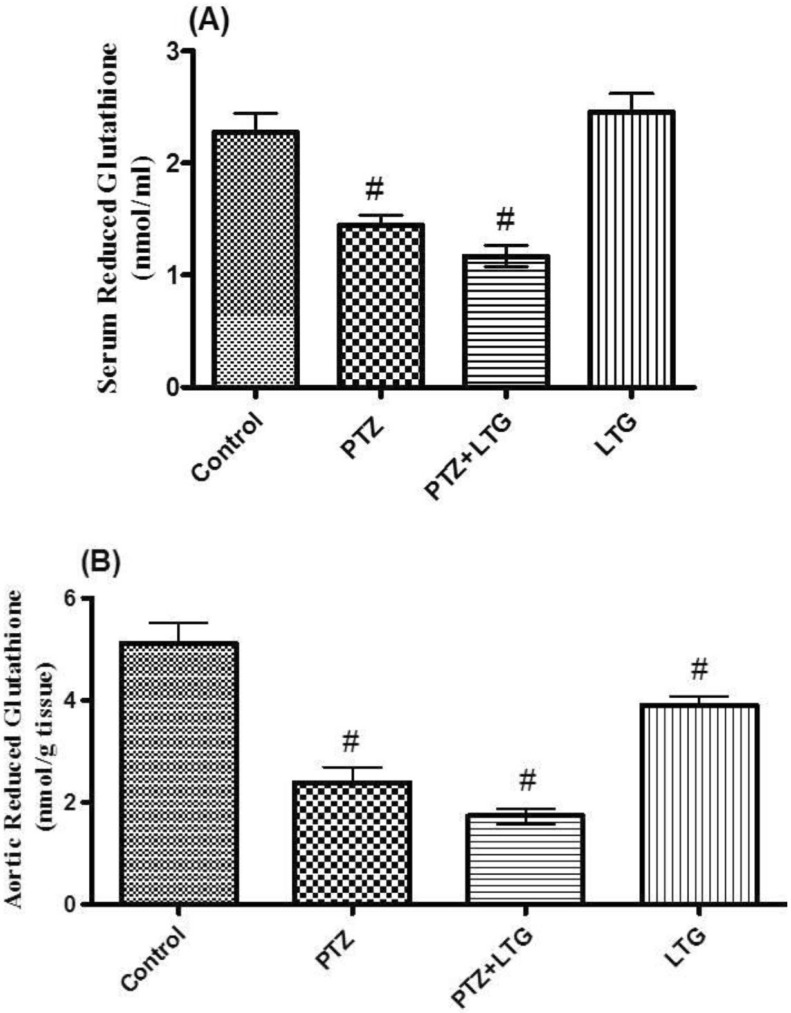 | Fig. 4(A, B) Serum and aortic reduced glutathione (GSH) levels in control rats, PTZ treated rats, PTZ+LTG and LTG treated rats. PTZ (pentylenetetrazole, 30 mg/kg), LTG (lamotrigine, 20 mg/kg). Data are expressed as mean±SD, number of animals=7. #p<0.05 compared to control group by analysis of variance (ANOVA), followed by Newman-Keuls multiple comparison test. |
Correlation between tHcy and both lipid profile and oxidative stress parameters
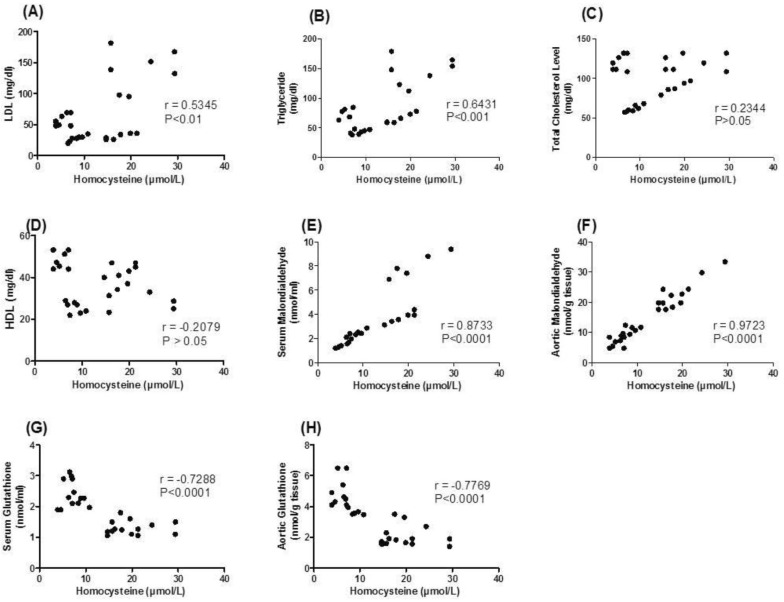 | Fig. 5(A-H) Pearson's correlation coefficients between serum total homocysteine (tHcy) concentration and both serum lipids and oxidative stress parameters in control, PTZ (Pentylenetetrazole) treated, PTZ+LTG (lamotrigine) and LTG treated rats. LDL, low density lipoprotein; HDL, high density lipoprotein. |
Effect of lamotrigine on endothelial dysfunction studies
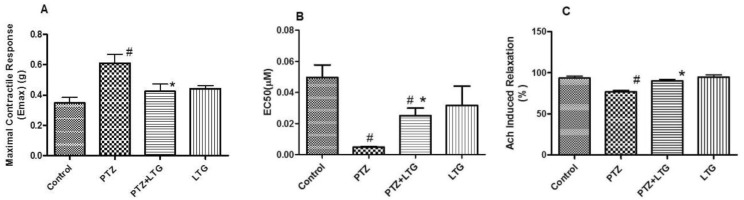 | Fig. 6(A) Maximal contractile response (E-max) induced by phenylephrine (PE). (B) Effective concentration 50 (EC50) of PE. (C) Relaxant responses to acetylcholine (Ach) in the endothelium-intact aortic rings of control rats, PTZ treated rats, PTZ+LTG and LTG treated rats. PTZ (pentylenetetrazole, 30mg/kg), LTG (lamotrigine, 20 mg/kg).Data are expressed as mean±SD, number of animals=7. #p<0.05 compared to control group, *p<0.05 compared to PTZ group by analysis of variance (ANOVA), followed by Newman-Keuls multiple comparison test. |
Effect of lamotrigine on histopathological changes
1. Brain histopathological and immunohistochemical staining changes
 | Fig. 7(rows A-D): A photomicrograph of CA3 region of the hippocampus: Row A, - Stained with H&E, ×540; (1) Control rat: notice that the pyramidal layer is formed of closely packed pyramidal cells with vesicular nuclei (↑). (2) Pentylenetetrazole (PTZ) treated rat: showing the presence of many deeply stained cells having pyknotic nuclei and perineuronal spaces (▸) an apparent decrease in the number of the pyramidal cells. (3) PTZ-Lamotrigine (LTG) treated rat: Pyramidal cells having vesicular nuclei and basophilic cytoplasm (↑). Few shrunken, darkly stained neurons are seen (▸) within the pyramidal layer. (4) LTG treated rat: nearly similar to control group (↑). Row B, Stained with Toluidine blue ×540: (1) Control rat: cytoplasm of the pyramidal cells appears studded with Nissl granules (↑). (2) PTZ treated rat: an apparent decrease in the Nissl granules of the pyramidal cells (↑). (3) LTG-PTZ treated rat: an apparent increase in the Nissl granules content of the pyramidal cells (↑) compared to that of PTZ treated rats. (4) LTG treated rat: picture nearly similar to control group. Row C, stained with Caspase-3 ×540; (1) Control rat: negative caspse-3 immuno- reaction in pyramidal cells. (2) PTZ treated rat: positive brownish caspase-3 immuno- reaction in pyramidal cells (↑). (3) PTZ-LTG treated rat: positive brownish caspase-3 immuno- reaction in few pyramidal cells (↑) when compared to PTZ group. (4): LTG treated rat: negative caspase-3 immuno- reaction in pyramidal cells. Row D, stained with Glial fibrillary acidic protein (GFAP) ×540. (1) Control rat: positive reaction for GFAP immuno-staining in few astrocytes (↑). (2) PTZ treated rat: an apparent increase in astrocytes (↑) size with longer cytoplasmic processes, number and GFAP immunoreactivity compared to control rats. (3) PTZ-LTG treated rat: an apparent decrease in the number of GFAP immunoreactive astrocytes (↑) compared to PTZ group. (4) LTG treated rat: few GFAP immunoreactive astrocytes (↑). |
Table 2
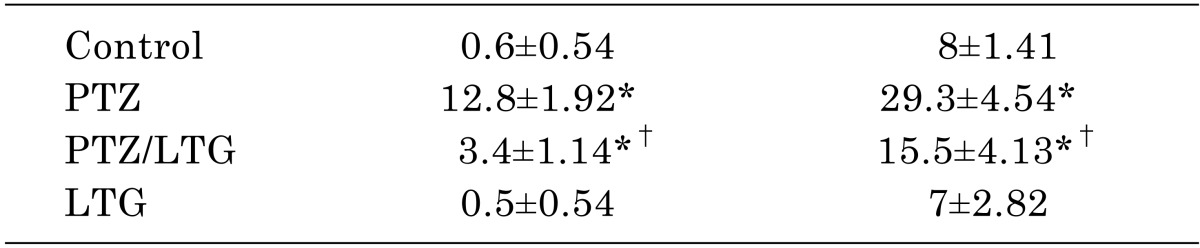
Data are expressed as mean±SD, number of animals (n)=7, PTZ (pentylenetetrazole, 30 mg/kg); LTG (lamotrigine, 20 mg/kg); GFAP, glial fibrillary acidic protein. *p<0.05 compared to control group, †p<0.05 compared to PTZ group by analysis of variance (ANOVA), followed by Newman-Keuls multiple comparison test.
2. Aortas and coronaries histopathological changes
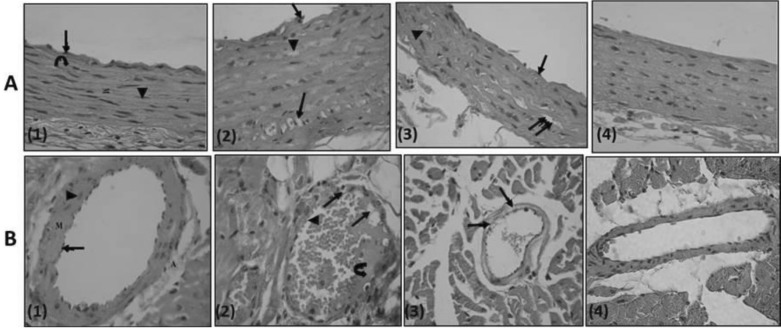 | Fig. 8(row A, B): Row A, A photomicrograph of a transverse section in a rat thoracic aorta (H&E, ×540); (1) Control rat: the tunica intima lined with endothelial cells (↑), tunica media (M) is relatively thick and formed mainly of wavy acidophilic elastic laminae (  ) and smooth muscle cells (▲). Notice tunica adventitia is thin. (2) PTZ treated rat: clumps of cells with darkly stained nuclei (↑) attached to the endothelium of tunica intima which is apparently irregular. Few of the smooth muscle cells of tunica media show foamy acidophilic cytoplasm with karyolitic nucleus (▲) or clearly vacuolated cytoplasm with peripheral flat pyknotic nucleus (↑↑). (3) PTZ-LTG treated rat: apparently regular tunica intima (↑). Most of the smooth muscle cells are nearly similar to control group, others show foamy acidophilic cytoplasm (↑↑) and few number shows vacuolated cytoplasm with deeply stained nucleus (▲). (4) LTG treated rat: tunicae of the aorta are nearly similar to the control group. Row B, A photomicrograph of a section a coronary artery of the heart (H&E, ×540); (1) Control rat: an intact endothelial lining (↑). The tunica media (M) is formed of smooth muscle fibres with rod shaped vesicular nuclei (▲). The adventitia (A) is formed of loose connective tissue. (2) PTZ treated rat: multiple vacuoles in the tunica intima and tunica media (↑). Notice adherence of blood elements to the endothelium (▲). Exudates are also seen ( ) and smooth muscle cells (▲). Notice tunica adventitia is thin. (2) PTZ treated rat: clumps of cells with darkly stained nuclei (↑) attached to the endothelium of tunica intima which is apparently irregular. Few of the smooth muscle cells of tunica media show foamy acidophilic cytoplasm with karyolitic nucleus (▲) or clearly vacuolated cytoplasm with peripheral flat pyknotic nucleus (↑↑). (3) PTZ-LTG treated rat: apparently regular tunica intima (↑). Most of the smooth muscle cells are nearly similar to control group, others show foamy acidophilic cytoplasm (↑↑) and few number shows vacuolated cytoplasm with deeply stained nucleus (▲). (4) LTG treated rat: tunicae of the aorta are nearly similar to the control group. Row B, A photomicrograph of a section a coronary artery of the heart (H&E, ×540); (1) Control rat: an intact endothelial lining (↑). The tunica media (M) is formed of smooth muscle fibres with rod shaped vesicular nuclei (▲). The adventitia (A) is formed of loose connective tissue. (2) PTZ treated rat: multiple vacuoles in the tunica intima and tunica media (↑). Notice adherence of blood elements to the endothelium (▲). Exudates are also seen ( ). (3) PTZ-LTG treated rat: few vacuolated cells are seen in tunica media (↑). (4) LTG treated rat: nearly intact tunicae. ). (3) PTZ-LTG treated rat: few vacuolated cells are seen in tunica media (↑). (4) LTG treated rat: nearly intact tunicae. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download