INTRODUCTION
METHODS
Cell culture
Plasmid and siRNA Transfection
Okadaic acid treatment
Protein extraction and Western blot analysis
Statistical analysis
RESULTS
Differential phosphorylation status of tau protein in the AD model cell line expressing the Swedish mutant form of amyloid precursor protein (APP-swe)
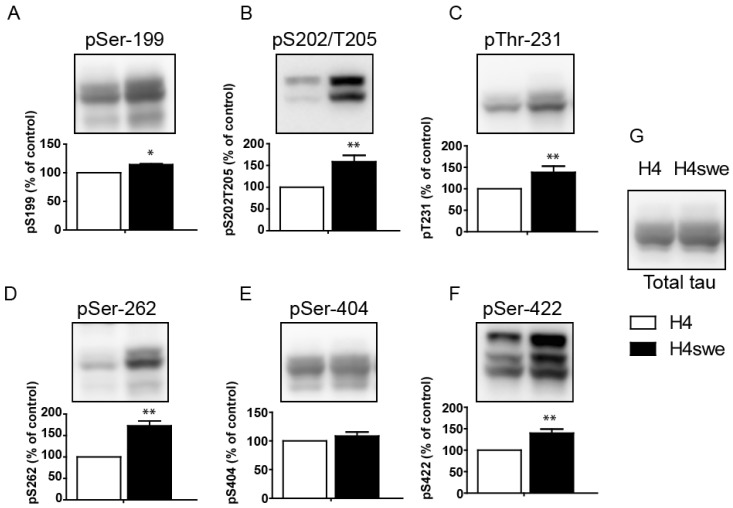 | Fig. 1Hyperphosphorylation of tau protein in the AD model cell line. A FLAG-tagged tau 441 expression construct was transfected into H4 wild-type cells (H4) or H4-swe cells, which express the Swedish mutant form of APP. The phosphorylation status was examined on (A) Ser-199, (B) Ser-202/Thr-205, (C) Thr-231, (D) Ser-262, (E) Ser-404, and (F) Ser-422 with the respective phospho-specific antibodies. Total tau protein was visualized with anti-FLAG antibody. Bar graphs show the phosphorylation levels of each phosphorylation site. Signals from H4-swe cells were normalized to values obtained for H4 wildtype cells. Statistical analyses were performed using a t-test (*p<0.001, **p<0.05). Data are the means±standard error of the mean (SEM, n=3). |
PP2A activity is required for the regulation of tau protein phosphorylation
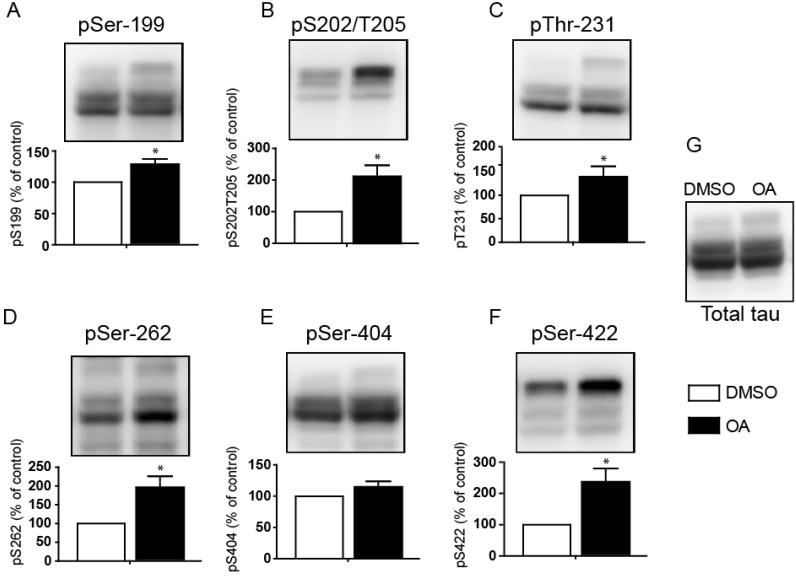 | Fig. 2PP2A-mediated regulation of the tau protein phosphorylation level. FLAG-tagged tau 441-transfected H4-swe cells were treated with 10 nM okadaic acid for 12 hours. The phosphorylation status was examined on (A) Ser-199, (B) Ser-202/Thr-205, (C) Thr-231, (D) Ser-262, (E) Ser-404, and (F) Ser-422 with a phospho-specific antibody corresponding to each site. Total tau protein was visualized with anti-FLAG antibody. Bar graphs show the phosphorylation levels of each phosphorylation site. Signals from H4-swe cells were normalized to values obtained for DMSO vehicle-treated cells. Statistical analyses were performed using a t-test (*p<0.001, **p<0.05). Data are the means±standard error of the mean (SEM, n=3). |
Regulatory B subunits are involved in site-specific regulation of tau phosphorylation
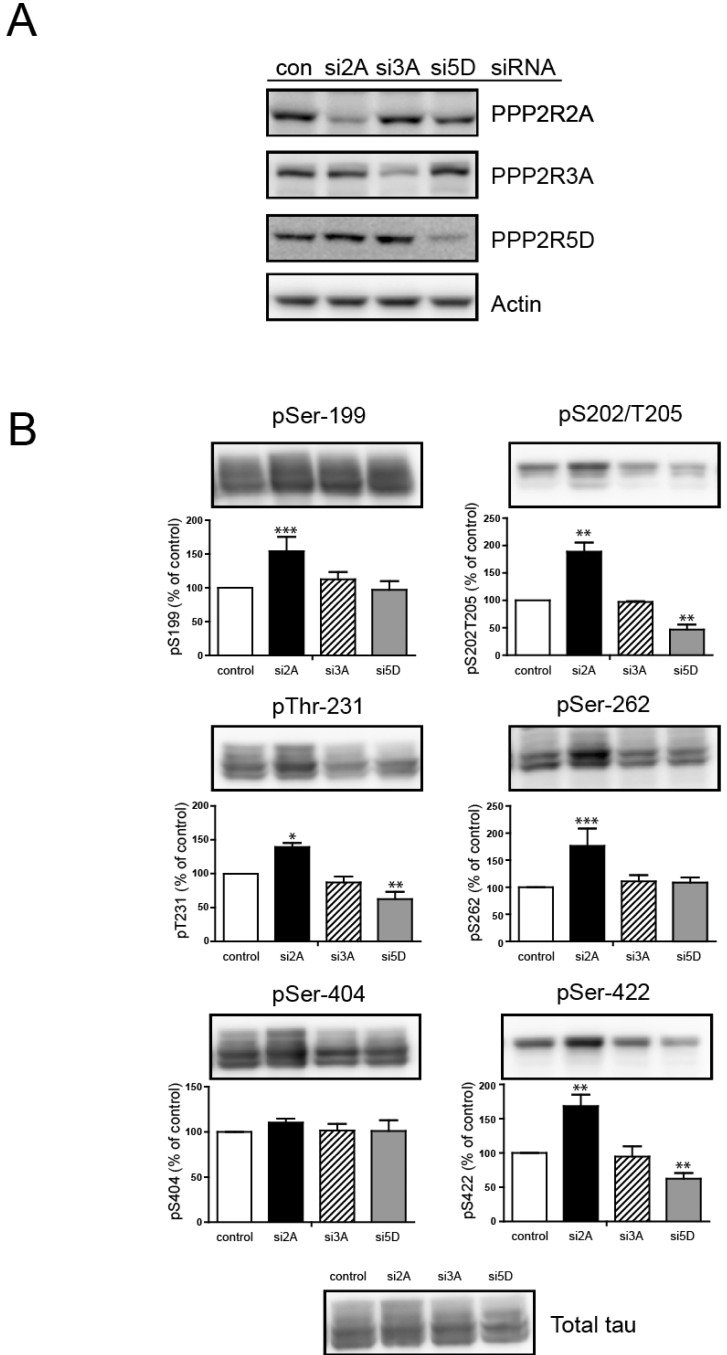 | Fig. 3Regulatory B subunits are involved in site-specific regulation of tau protein phosphorylation. B regulatory subunits were knocked down with siRNA specific to each B subunit; PPP2R2A siRNA (si2A), PPP2R3A siRNA (si3A), or PPP2R5D siRNA (si5D) was transfected into H4-swe cells. (A) The expression level of each B subunit was measured with antibodies corresponding to each B subunit. (B) Phosphorylation status analysis was performed with immunoblotting for each site as indicated. Bar graphs show the phosphorylation level for each site normalized to control siRNA-treated H4 cells. Statistical analyses were performed using a t-test (*p<0.001, **p<0.01, ***p<0.05). Data are the means±standard error of the mean (SEM, n=3). |
The PPP2R5D subunit regulates tau phosphorylation via Akt-mediated GSK3β activation
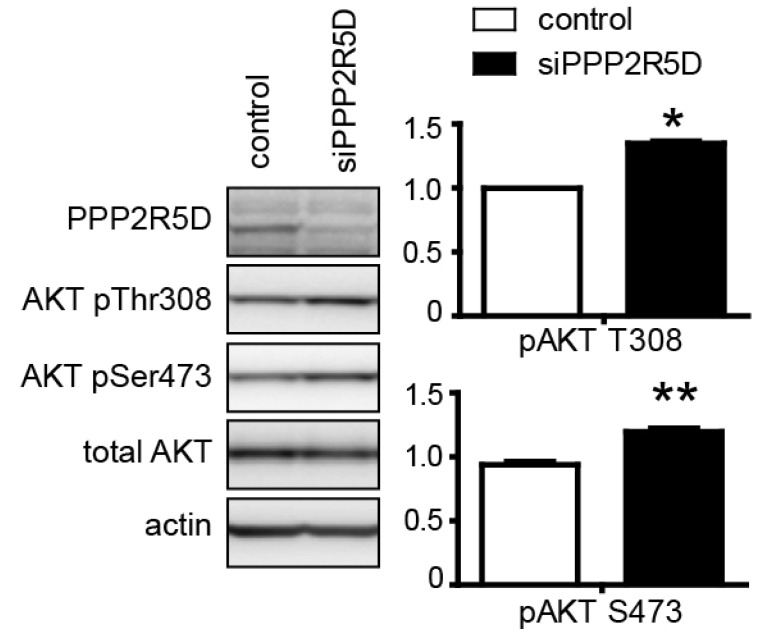 | Fig. 4PP2A with the PPP2R5D subunit regulates the AKT-GSK3β signaling pathway. H4 cells were transfected with control siRNA or PPP2R5D siRNA (left panel), or a control vector or a PPP2R5D expression construct (right panel) as indicated. The status of inhibitory phosphorylation at Ser-9 of GSK3β was visualized with a phospho-specific antibody. H4 cells were transfected with siRNA specific for the PPP2R5D subunit, and then the status of activating phosphorylation at Thr-308 and Ser-473 was visualized with a phospho-specific antibody corresponding to each site. The bar graph shows the relative signal intensity of siRNA-transfected samples compared to the control siRNA-transfected sample. Statistical analyses were performed using a t-test (*p<0.001, **p<0.01). Data are the means±standard error of the mean (SEM, n=3). |
Identification of regulatory B subunit-specific novel phosphorylation sites in tau protein
Table 1

FLAG-tagged tau 441 was transfected into H4 cells with control siRNA, PPP2R2A siRNA (si2A), PPP2R3A siRNA (si3A), or PPP2R5D siRNA (si5D). Tau proteins were immunoprecipitated with anti-FLAG antibody 48 hours after transfection. Tau protein was in-gel digested with trypsin for LC-MS analysis. LC-MS analysis was repeated for four times using immunoprecipitated tau obtained from four independent experiments. The number indicates the frequency of each phospho-peptide from four independent experiments.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download