INTRODUCTION
METHODS
Nodose neuron dissociation
Electrical measurements
Drugs & ginger extraction
Data analysis
RESULTS
Inhibition of 5-HT-induced current by ginger extract
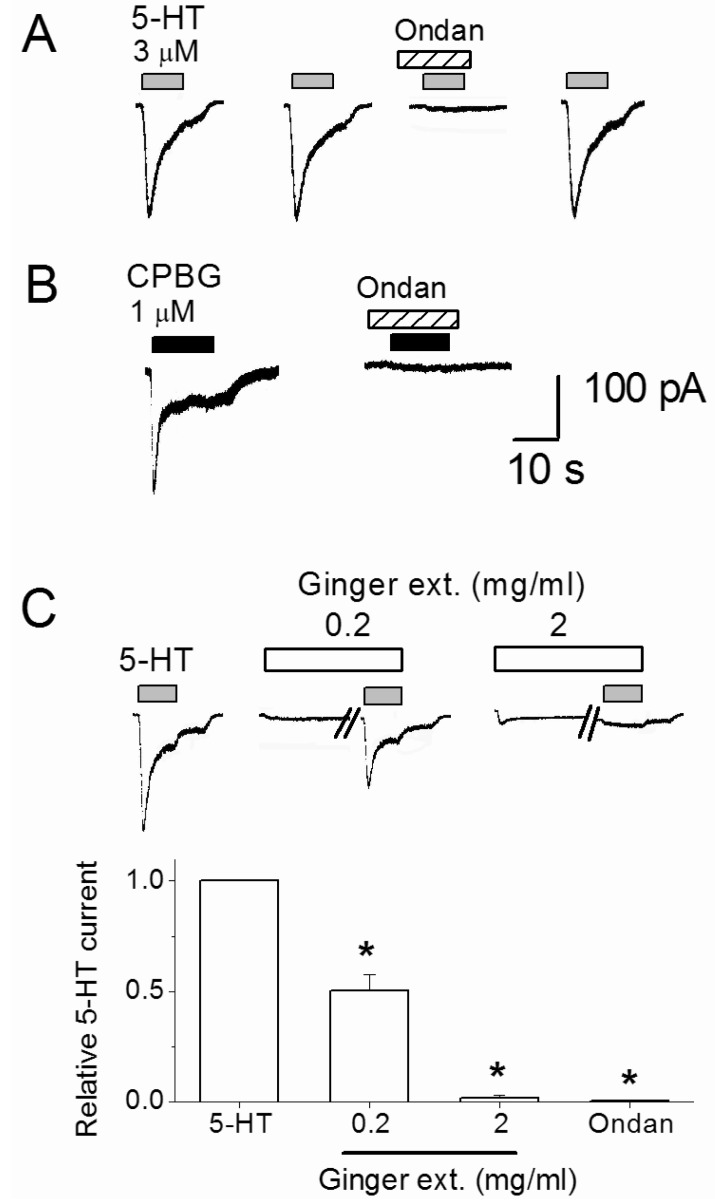 | Fig. 2Inhibition of 5-HT-evoked current by ginger dry extract (Ginger ext.) and ondansetron (Ondan). (A) Serotonin (3 µM) was repeatedly applied with 4 min intervals with or without 5-HT3 receptor antagonist ondansetron (10 nM). (B) CPBG-evoked current was blocked by 10 nM ondansetron. (C) The traces show that 5-HT-evoked currents were suppressed by 0.2 and 2 mg/ml ginger dry extract. Ginger extract was pre-applied 2 min before co-application with 3 µM 5-HT. The diagram shows summary of average 5-HT current change by 0.2 and 2 mg/ml ginger extract and 10 nM ondansetron treatments. *Significant difference from control (p≤0.01). Each column and vertical lines represent the mean±SEM. |
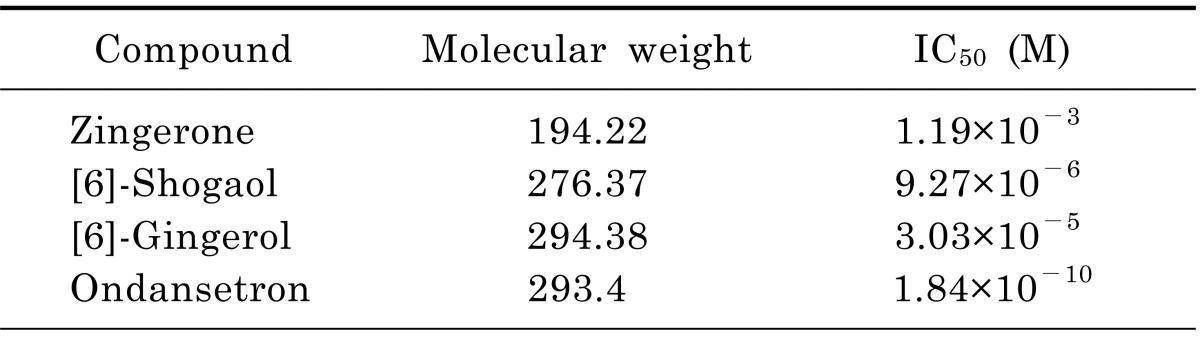
Inhibition of 5-HT current by ginger constituents
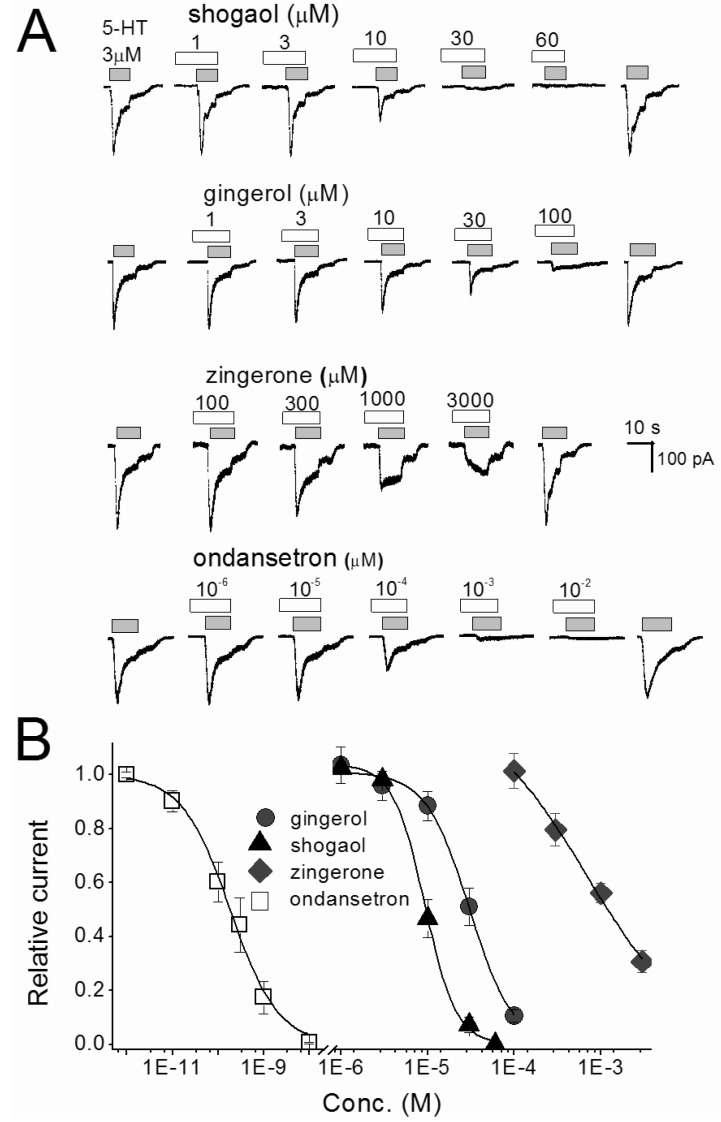 | Fig. 3Concentration-inhibition relationships for single compounds of ginger and ondansetron in nodose ganglion neurons. (A) The traces shown are inhibition of 3 µM 5-HT-evoked current by [6]-shogaol, [6]-gingerol, zingerone and ondansetron. The traces were taken from different set of neurons. Various concentrations of reagents were pretreated 30 sec, and then co-application with 5-HT. (B) Represents the concentration-inhibition relationship of [6]-shogaol, [6]-gingerol, zingerone and ondansetron. Each point is the average of 4~7 neurons. |
Pungent ginger constituents act as non-competitive antagonists
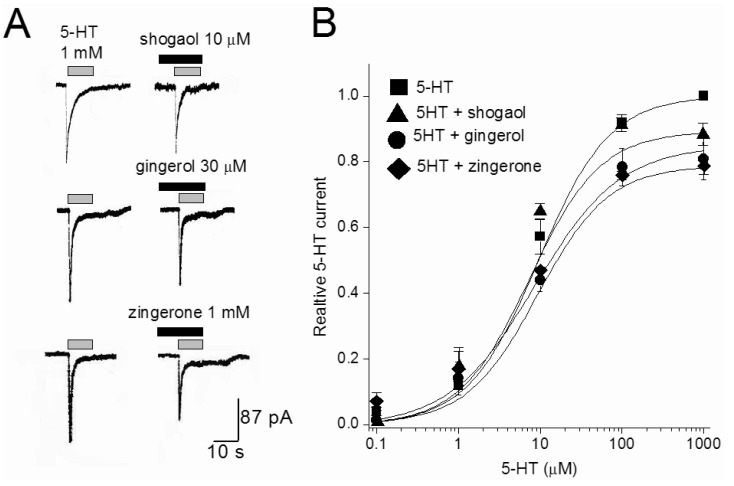 | Fig. 4The effect of [6]-shogaol, [6]-gingerol, and zingerone on 5-HT concentration-response curves. (A) Representative currents traces activated by 1 mM 5-HT in the presence or absence of [6]-shogaol (10 µM), [6]-gingerol (30 µM), and zingerone (1 mM). Various concentrations of reagents were pretreated 30 sec, and then co-application with 5-HT. (B) Concentration-response relationship for 5-HT in the absence and presence of 10 µM [6]-shogaol, 30 µM [6]-gingerol, and 1 mM zingerone. The current amplitude is normalized to the current activated by 1 mM 5-HT. Data points are the mean±S.E.M. (n=4~6). The curve is the best fit of the data to the Hill equation described in methods. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download