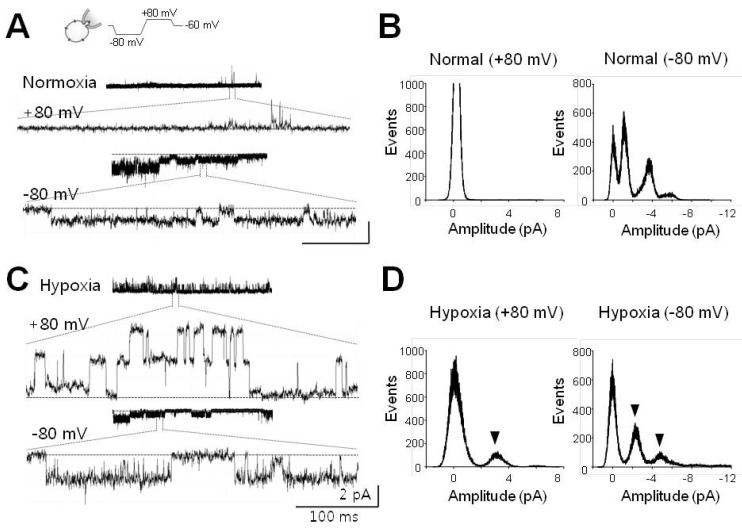
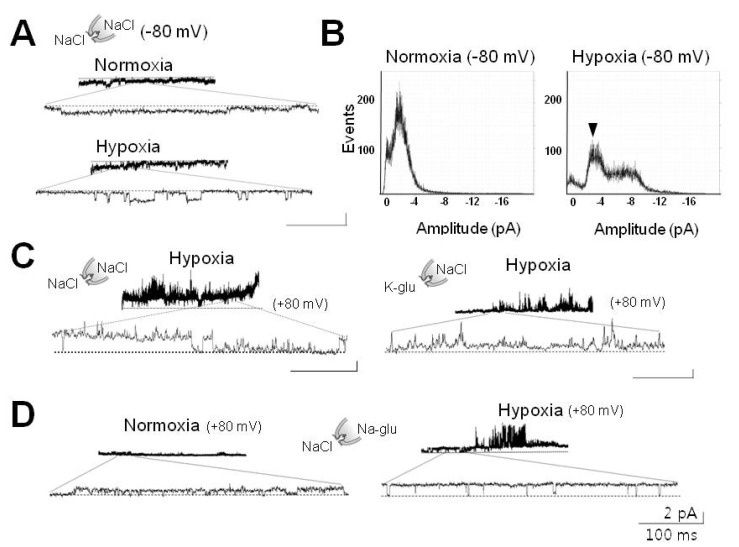
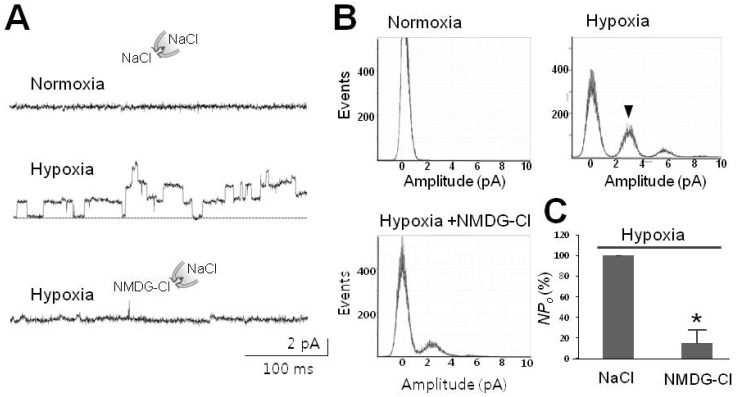
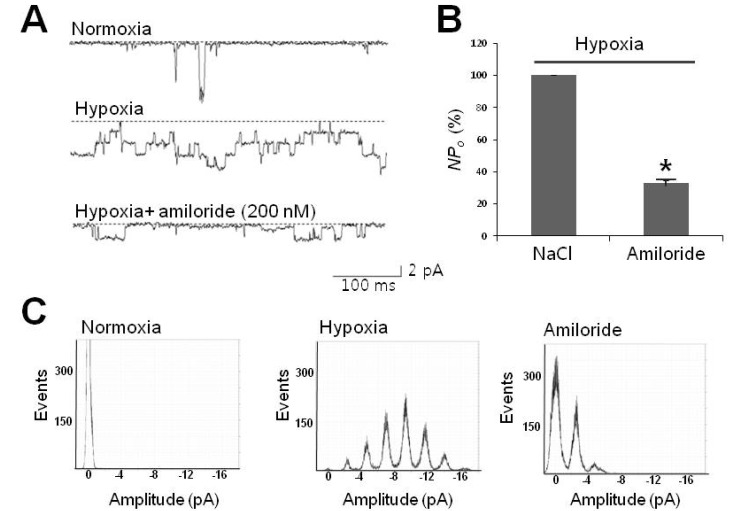
1. Cross JL, Meloni BP, Bakker AJ, Lee S, Knuckey NW. Modes of neuronal calcium entry and homeostasis following cerebral ischemia. Stroke Res Treat. 2010; 2010:316862. PMID:
21052549.

2. Fung ML. Role of voltage-gated Na
+ channels in hypoxia-induced neuronal injuries. Clin Exp Pharmacol Physiol. 2000; 27:569–574. PMID:
10901384.
3. Ratan RR, Siddiq A, Smirnova N, Karpisheva K, Haskew-Layton R, McConoughey S, Langley B, Estevez A, Huerta PT, Volpe B, Roy S, Sen CK, Gazaryan I, Cho S, Fink M, LaManna J. Harnessing hypoxic adaptation to prevent, treat, and repair stroke. J Mol Med (Berl). 2007; 85:1331–1338. PMID:
18043901.

4. Conrad PW, Conforti L, Kobayashi S, Beitner-Johnson D, Rust RT, Yuan Y, Kim HW, Kim RH, Seta K, Millhorn DE. The molecular basis of O
2-sensing and hypoxia tolerance in pheochromocytoma cells. Comp Biochem Physiol B Biochem Mol Biol. 2001; 128:187–204. PMID:
11207433.
5. Conforti L, Millhorn DE. Selective inhibition of a slow-inactivating voltage-dependent K
+ channel in rat PC12 cells by hypoxia. J Physiol. 1997; 502:293–305. PMID:
9263911.
6. Zhu WH, Conforti L, Czyzyk-Krzeska MF, Millhorn DE. Membrane depolarization in PC-12 cells during hypoxia is regulated by an O
2-sensitive K
+ current. Am J Physiol. 1996; 271:C658–C665. PMID:
8770007.
7. Conforti L, Bodi I, Nisbet JW, Millhorn DE. O
2-sensitive K
+ channels: role of the Kv1.2 -subunit in mediating the hypoxic response. J Physiol. 2000; 524:783–793. PMID:
10790158.
8. Kim D. K
+ channels in O
2 sensing and postnatal development of carotid body glomus cell response to hypoxia. Respir Physiol Neurobiol. 2013; 185:44–56. PMID:
22801091.
9. López-López J, González C, Ureña J, López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989; 93:1001–1015. PMID:
2738574.

10. López-López JR, González C, Pérez-García MT. Properties of ionic currents from isolated adult rat carotid body chemoreceptor cells: effect of hypoxia. J Physiol. 1997; 499:429–441. PMID:
9080372.

11. Ji HL, Benos DJ. Degenerin sites mediate proton activation of deltabetagamma-epithelial sodium channel. J Biol Chem. 2004; 279:26939–26947. PMID:
15084585.
12. Wesch D, Miranda P, Afonso-Oramas D, Althaus M, Castro-Hernández J, Dominguez J, Morty RE, Clauss W, González-Hernández T, Alvarez de la Rosa D, Giraldez T. The neuronalneuronal-specific SGK1.1 kinase regulates δ-epithelial Na
+ channel independently of PY motifs and couples it to phospholipase C signaling. Am J Physiol Cell Physiol. 2010; 299:C779–C790. PMID:
20631247.
13. Seta KA, Spicer Z, Yuan Y, Lu G, Millhorn DE. Responding to hypoxia: lessons from a model cell line. Sci STKE. 2002; 2002:re11. PMID:
12189251.

14. Abe E, Fujiki M, Nagai Y, Shiqi K, Kubo T, Ishii K, Abe T, Kobayashi H. The phosphatidylinositol-3 kinase/Akt pathway mediates geranylgeranylacetone-induced neuroprotection against cerebral infarction in rats. Brain Res. 2010; 1330:151–157. PMID:
20206146.

15. Tong Q, Gamper N, Medina JL, Shapiro MS, Stockand JD. Direct activation of the epithelial Na
+ channel by phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate produced by phosphoinositide 3-OH kinase. J Biol Chem. 2004; 279:22654–22663. PMID:
15028718.
16. Chen Y, Shi G, Xia W, Kong C, Zhao S, Gaw AF, Chen EY, Yang GP, Giaccia AJ, Le QT, Koong AC. Identification of hypoxia-regulated proteins in head and neck cancer by proteomic and tissue array profiling. Cancer Res. 2004; 64:7302–7310. PMID:
15492250.

17. Lebowitz J, Edinger RS, An B, Perry CJ, Onate S, Kleyman TR, Johnson JP. Ikappab kinase-beta (ikkbeta) modulation of epithelial sodium channel activity. J Biol Chem. 2004; 279:41985–41990. PMID:
15292220.
18. Choi SW, Ahn JS, Kim HK, Kim N, Choi TH, Park SW, Ko EA, Park WS, Song DK, Han J. Increased expression of atp-sensitive K+ channels improves the right ventricular tolerance to hypoxia in rabbit hearts. Korean J Physiol Pharmacol. 2011; 15:189–194. PMID:
21994476.

19. Wood JN, Boorman JP, Okuse K, Baker MD. Voltage-gated sodium channels and pain pathways. J Neurobiol. 2004; 61:55–71. PMID:
15362153.

20. Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005; 57:397–409. PMID:
16382098.

21. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002; 82:735–767. PMID:
12087134.

22. Hammarström AK, Gage PW. Hypoxia and persistent sodium current. Eur Biophys J. 2002; 31:323–330. PMID:
12202908.

23. Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997; 386:173–177. PMID:
9062189.

24. Alexander SPH, Mathie A, Peters JA. Guide to receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011; 164(Suppl 1):S1–S324. PMID:
22040146.

25. Ohbuchi T, Sato K, Suzuki H, Okada Y, Dayanithi G, Murphy D, Ueta Y. Acid-sensing ion channels in rat hypothalamic vasopressin neurons of the supraoptic nucleus. J Physiol. 2010; 588:2147–2162. PMID:
20442265.

26. Chu XP, Miesch J, Johnson M, Root L, Zhu XM, Chen D, Simon RP, Xiong ZG. Proton-gated channels in PC12 cells. J Neurophysiol. 2002; 87:2555–2561. PMID:
11976391.

27. Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. . Physiol Rev. 1997; 77:359–396. PMID:
9114818.

28. Kellenberger S, Hoffmann-Pochon N, Gautschi I, Schneeberger E, Schild L. On the molecular basis of ion permeation in the epithelial Na
+ channel. J Gen Physiol. 1999; 114:13–30. PMID:
10398689.
29. Anantharam A, Palmer LG. Determination of epithelial Na
+ channel subunit stoichiometry from single-channel conductances. J Gen Physiol. 2007; 130:55–70. PMID:
17562820.
30. Ismailov II, Berdiev BK, Bradford AL, Awayda MS, Fuller CM, Benos DJ. Associated proteins and renal epithelial Na
+ channel function. J Membr Biol. 1996; 149:123–132. PMID:
8834119.
31. Kelly O, Lin C, Ramkumar M, Saxena NC, Kleyman TR, Eaton DC. Characterization of an amiloride binding region in the alpha-subunit of ENaC. Am J Physiol Renal Physiol. 2003; 285:F1279–F1290. PMID:
12928313.
32. Ma HP, Al-Khalili O, Ramosevac S, Saxena S, Liang YY, Warnock DG, Eaton DC. Steroids and exogenous γ-ENaC subunit modulate cation channels formed by α-ENaC in human B lymphocytes. J Biol Chem. 2004; 279:33206–33212. PMID:
15187080.

33. Ma HP, Chou CF, Wei SP, Eaton DC. Regulation of the epithelial sodium channel by phosphatidylinositides: experiments, implications, and speculations. Pflugers Arch. 2007; 455:169–180. PMID:
17605040.

34. Yamashima T, Saido TC, Takita M, Miyazawa A, Yamano J, Miyakawa A, Nishijyo H, Yamashita J, Kawashima S, Ono T, Yoshioka T. Transient brain ischaemia provokes Ca
2+, PIP
2 and calpain responses prior to delayed neuronal death in monkeys. Eur J Neurosci. 1996; 8:1932–1944. PMID:
8921284.
35. Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, Gabriel SE. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002; 277:8338–8345. PMID:
11756432.