Abstract
We recently reported a Philyra pisum lectin (PPL) that exerts mitogenic effects on human lymphocytes, and its molecular characterization. The present study provides a more detailed characterization of PPL based on the results from a monosaccharide analysis indicating that PPL is a glycoprotein, and circular dichroism spectra revealing its estimated α-helix, β-sheet, β-turn, and random coil contents to be 14.0%, 39.6%, 15.8%, and 30.6%, respectively. These contents are quite similar to those of deglycosylated PPL, indicating that glycans do not affect its intact structure. The binding properties to different pathogen-associated molecular patterns were investigated with hemagglutination inhibition assays using lipoteichoic acid from Gram-positive bacteria, lipopolysaccharide from Gram-negative bacteria, and both mannan and β-1,3-glucan from fungi. PPL binds to lipoteichoic acids and mannan, but not to lipopolysaccharides or β-1,3-glucan. PPL exerted no significant antiproliferative effects against human breast or bladder cancer cells. These results indicate that PPL is a glycoprotein with a lipoteichoic acid or mannan-binding specificity and which contains low and high proportions of α-helix and β-structures, respectively. These properties are inherent to the innate immune system of P. pisum and indicate that PPL could be involved in signal transmission into Gram-positive bacteria or fungi.
Invertebrates, like vertebrates, possess innate immune defense systems against bacterial, fungal, and viral pathogens [1,2]. Pathogen-associated molecular patterns (PAMPs) such as lipoteichoic acid (LTA), lipopolysaccharide (LPS), mannan, β-1,3-glucan, lipoproteins, and peptidoglycan have molecular sequences that are consistently found on the surfaces of pathogenic organisms [3,4]. PAMPs are recognized by pattern-recognition proteins (PRPs), which are secreted molecules that circulate in the hemolymph of invertebrates that lack acquired immune systems and bind to pathogens, thereby initiating signals leading to the release of pathogens [5,6].
Lectins are carbohydrate-binding proteins of nonimmune origin [7,8], and are important PRPs found in hemolymph along with coagulation factors, protease inhibitors, antimicrobial substances, and other proteins in invertebrates [1,2,9]. Lectins bind to specific carbohydrates on the surfaces of pathogens to activate complementary antipathogenic pathways [9,10].
Numerous studies of the structure of plant lectins and their carbohydrate-binding regions have revealed that they consist mainly of β-structures [11]. However, little information on the structure and function of marine invertebrates has been reported [12].
The marine crab Philyra pisum belongs to the family Leucosiidae and is distributed throughout the muddy areas of the west coast of South Korea. Our previous investigation indicated that P. pisum lectin (PPL), which has a molecular mass of 24,060 Da and is obtained from the hemolymph of P. pisum, exhibits hemagglutination and antiproliferative activities in human lung cancer cells [13]. The molecular characterization of PPL including its molecular mass, pI value, amino acid sequences, amino acid composition, and effects of pH, temperature, and metal ions on its activity as well as mitogenic activities on human lymphocytes were also investigated [14].
In the present study we investigated the characterization of PPL, such as by analyzing its monosaccharide composition, its secondary structure, and the structural effect of glycan, as well as its possibility of binding to different PAMPs and its antiproliferative effects on human cancer cells.
Hemolymph was prepared from P. pisum and the PPL was purified from this hemolymph as described previously [13,14]. The concentration of the purified lectin was determined by the Bradford assay [15] using bovine serum albumin (Bio-Rad, Hercules, CA, USA) as the standard. The hemagglutination activity was assayed using native erythrocytes at 2% according to a previously described method [13,16].
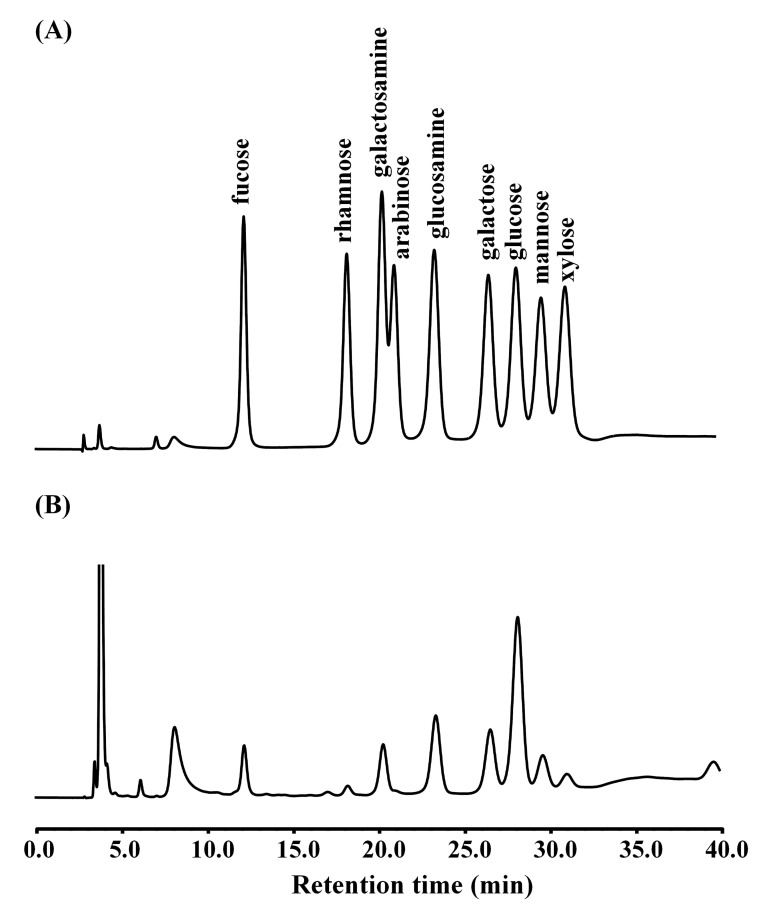
PPL (0.1 mg) was incubated with 2 M trifluoroacetic acid (Sigma, St. Louis, MO, USA) for 4 h at 100℃ to obtain the neutral and amino sugars. Monosaccharides were separated using high-pH anion-exchange chromatography (HPAEC) with a pulsed amperometric detector (PAD) (Dionex, Sunnyvale, CA, USA) equipped with a CarboPac™ PA10 column (4×250 mm) (Dionex) and an AminoTrap™ column (4×20 mm) (Dionex) working at an isocratic concentration of 20 mM NaOH (Fisher Scientific, Fair Lawn, NJ, USA) and at a flow rate of 0.5 ml/min at room temperature. The concentration of each monosaccharide was quantified from a calibration curve constructed using a standard mixture containing twofold dilutions from 800 pmol of each monosaccharide. The monosaccharides used in this study (e.g., fucose, galactose, glucose, mannose, galactosamine, glucosamine, xylose, and arabinose) were all purchased from Sigma.
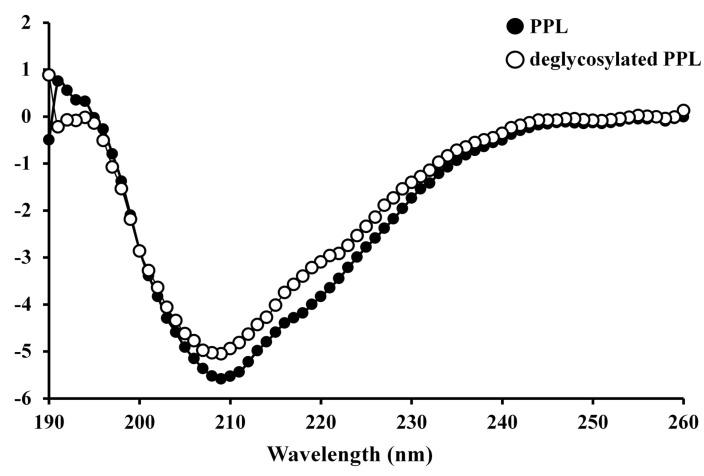
Circular dichroism (CD) spectrum analysis was performed in the far-UV region (190~260 nm) using a spectrophotometer (J-715, Jasco, Tokyo, Japan). The temperature was maintained at 20℃ when analyzing samples (0.1 mg/ml) in 20 mM ammonium bicarbonate (pH 8.0). The scan speed was 100 nm/min, the response time was 4 sec, and the bandwidth was 1.0 nm. The CD spectrum was plotted on a millidegree ellipticity scale.
The PPL glycans were chemically released using trifluoromethanesulfonic acid (TFMS) contained in the Glycoprofile IV chemical deglycosylation kit (Sigma), and the reaction was performed according to the manufacturer's instructions. The deglycosylated PPL was lyophilized and stored at -20℃.
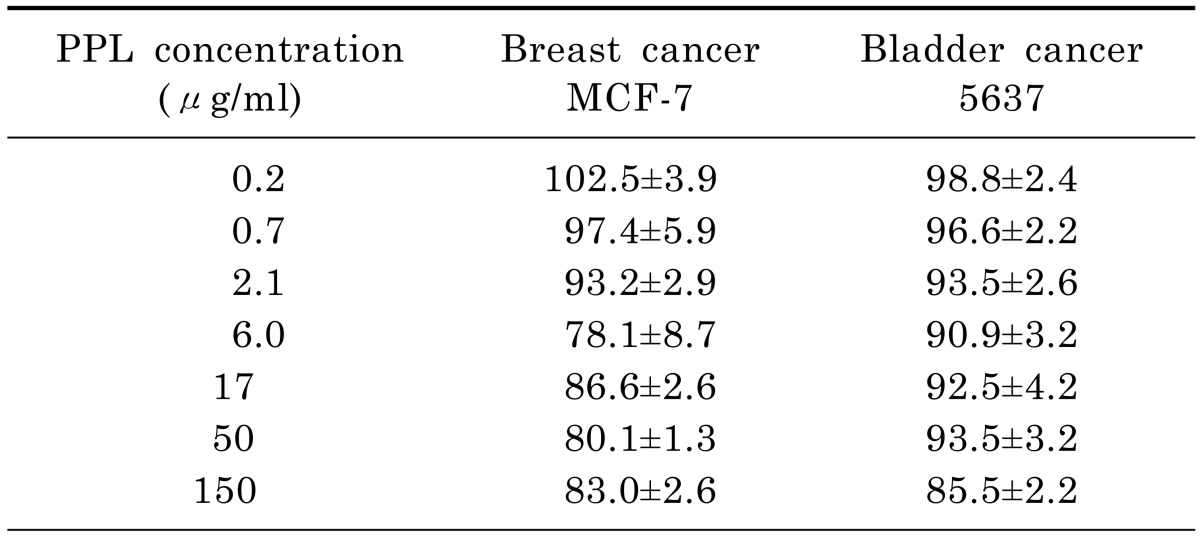
Human breast cancer MCF-7 and human bladder cancer 5637 cell lines were purchased from Korean Cell Line Bank. Cells (8×103 cells/0.1 ml/well) were incubated with PPL (100 µl) in 96-well culture plates for 72 h in a humidified atmosphere of 5% CO2 at 37℃. The percentage viability of the cancer cells was determined using the 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-8, Dojindo, Kumamoto, Japan) assay according to a modified version of Tominaga's method [17], and quantified as {(absorbance of treated cells)/(absorbance of untreated cells)}×100.
The purified PPL exhibited the same hemagglutination activity as reported previously [13] (data not shown). The composition of each monosaccharide in the purified PPL was identified by comparing the retention time of the monosaccharide peak in the standard obtained using the HPAEC-PAD method (Fig. 1). The monosaccharide ratio was calculated as a percentage based of the moles of monosaccharide per mole of PPL. As shown in Fig. 1, the monosaccharides of PPL (50 pmol) mainly comprised glucosamine (166.8±2.2 pmol, mean±SD; 35.3%), galactose (145.4±0.3 pmol, 30.8%), galactosamine (68.5±0.5 pmol, 14.5%), fucose (54.4±1.3 pmol, 11.5%), and mannose (37.3±1.3 pmol, 7.9%).
We investigated the carbohydrate-binding specificity of PPL using a competitive inhibition assay with PAMPs at various concentrations. As indicated in Table 1, the hemagglutination activity of PPL was inhibited by all kinds of LTAs from the various Gram-positive bacteria used in this study, including those from Staphylococcus aureus (8 µg/ml), Streptococcus pyogenes (31 µg/ml), Streptococcus faecalis (4 µg/ml), and Bacillus subtilis (2 µg/ml), as well as by mannan from Saccharomyces cerevisiae (31 µg/ml). However, LPSs from Escherichia coli 055:B5, E. coli 0111:B4, and Klebsiella pneumoniae or laminarin from Laminaria digitata did not lead to inhibition even when they were applied at concentrations up to 500 µg/ml.
The CD spectrum of PPL in its native form was characterized by minima at 209 nm and 217 nm and a maximum at 191 nm, with a negative-to-positive crossover at 194 nm, which manifested as ellipticity (Fig. 2). The secondary structure of PPL was determined using the protocol reported by Yang et al. [18], which revealed that its estimated α-helix, β-sheet, β-turn, and random coil contents were 14.0%, 39.6%, 15.8%, and 30.6%, respectively; the corresponding contents of these structures in deglycosylated PPL were calculated as 13.7%, 40.0%, 15.8%, and 30.5%, respectively.
The antiproliferation against human breast and bladder cancer cells was slightly decreased, but the percentage viabilities for both types of cell still exceeded 80% of that for the untreated control cells. Accordingly, PPL exerted no significant antiproliferative effects on human breast cancer MCF-7 cells or human bladder 5637cells (Table 2).
The results from the hemagglutination inhibition assays indicated that PPL binds with high specificity to both LTA and mannan-despite them sharing no common structures-but not to LPS or β-1,3-glucan. LTA and LPS are the cell wall components of Gram-positive and Gram-negative bacteria, respectively, while mannan and β-1,3-glucan are constituents of fungal cell walls [4,19,20].
There have been reports of a mannose (mannan)-binding lectin that specifically binds to bacterial LTA through either a carbohydrate recognition domain or another part of the domain [21,22]. The present results showing differential binding to microbial components such as LTA or mannan indicated that different regions of PPL probably participate in binding to microbial components in a similar way to previously reported lectins [23]. Furthermore, our data show that recognition is the initiation process in various immune responses in this crab species, such as prophenoloxidase activation, phagocytosis, nodule formation, and encapsulation [2,3].
The monosaccharide analysis revealed that PPL is a glycoprotein composed of N-acetylglucosamine, galactose, N-acetylgalactosamine, fucose, and mannose, since of de-N-acetylation of monosaccharides such as N-acetylgalactosamine and N-acetylglucosamine occurred during acid hydrolysis. Studies investigating the effect of glycosylation on glycoprotein structure have mainly used completely deglycosylated proteins generated through chemical degradation with reagents such as TFMS [24]. However, the present study has demonstrated that the secondary-structure contents of deglycosylated PPL are quite similar to those of intact PPL, mean that glycans exert almost no effect on the intact structure of PPL.
The proportions of α-helix (4~61%) and β-sheet (6~57%) structures vary widely in marine invertebrate lectin [25-29]. In contrast, the PAMP-binding domain of PRP obtained from the hemolymph of a pyralid moth reportedly consists primarily of α-helix, with only a small amount of β-sheet [30]. PPL contains a relatively low proportion of α-helix, which is broadly consistent with reports for legume lectins (as also determined using the protocol of Yang et al.) including a low proportion (less than 30%) of α-helix and a high proportion (more than 60%) of β-sheet [11,31,32].
There have been numerous reports on the structure and carbohydrate-binding specificity of plant lectins. However, little information on lectins isolated from marine invertebrates has been reported. Many lectins exert antiproliferative effects against human cancer cells, but the mechanisms of action are not fully understood [33]. These antiproliferative activities are probably related to the type and linkage of the glycoconjugate on the surface membranes of cancer cells [8].
In conclusion, the present results indicate that PPL is a glycoprotein whose secondary structure includes a relatively low proportion (14.0%) of α-helix and a high proportion (55.4%) of β-structure, and that this is not affected by glycans. PPL binds to both bacterial LTA and fungal mannan. Our previous study showed that PPL exerts antiproliferative effects on human lung cancer cells [13]; in contrast, the present study found no such effects on human breast and bladder cancer cells. These properties are inherent to the innate immune system of P. pisum and indicate that PPL could be involved in signal transmission into Gram-positive bacteria or fungi. Further investigations including a detailed structural analysis and determination of the binding constant to LTA or mannan as well as the binding sites to PAMPs of PPL are underway in our laboratory.
ACKNOWLEDGEMENTS
This study was supported by the Rural Development Administration (PJ0098432013), Republic of Korea and the Chung-Ang University Excellent Student Scholarship.
References
1. Medzhitov R, Janeway CA Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997; 91:295–298. PMID: 9363937.

2. Lee SY, Söderhäll K. Early events in crustacean innate immunity. Fish Shellfish Immunol. 2002; 12:421–437. PMID: 12194453.
3. Medzhitov R, Janeway C Jr. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000; 173:89–97. PMID: 10719670.

4. Kurata S, Ariki S, Kawabata S. Recognition of pathogens and activation of immune responses in Drosophila and horseshoe crab innate immunity. Immunobiology. 2006; 211:237–249. PMID: 16697917.

5. Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999; 284:1313–1318. PMID: 10334979.

6. Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002; 20:197–216. PMID: 11861602.

7. Lis H, Sharon N. Lectins as molecules and as tools. Annu Rev Biochem. 1986; 55:35–67. PMID: 3527046.

8. Sharon N, Lis H. Lectins-proteins with a sweet tooth: functions in cell recognition. Essays Biochem. 1995; 30:59–75. PMID: 8822149.
9. Kawabata S, Iwanaga S. Role of lectins in the innate immunity of horseshoe crab. Dev Comp Immunol. 1999; 23:391–400. PMID: 10426430.

10. Kilpatrick DC. Animal lectins: a historical introduction and overview. Biochim Biophys Acta. 2002; 1572:187–197. PMID: 12223269.

11. Loris R, Hamelryck T, Bouckaert J, Wyns L. Legume lectin structure. Biochim Biophys Acta. 1998; 1383:9–36. PMID: 9546043.

12. Vasta GR, Ahmed H, Odom EW. Structural and functional diversity of lectin repertoires in invertebrates, protochordates and ectothermic vertebrates. Curr Opin Struct Biol. 2004; 14:617–630. PMID: 15465324.

13. Kim HH, Jun JI, Kim BS, Cho DH, Min TH, Kim YJ, Ryu CS. Lectin protein prepared from Korean marine crab Philyra pisum, process for preparing the same and the use thereof. US Patent. 07015313. 2006.
14. Na JC, Park BT, Chung WH, Kim HH. Molecular characterization and mitogenic activity of a lectin from purse crab Philyra pisum. Korean J Physiol Pharmacol. 2011; 15:241–244. PMID: 21994481.
15. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254. PMID: 942051.

16. Kim BS, Oh KT, Cho DH, Kim YJ, Koo WM, Kong KH, Kim HH. A sialic acid-binding lectin from the legume Maackia fauriei: comparison with lectins from M. amurensis. Plant Sci. 2004; 167:1315–1321.
17. Tominaga H, Ishiyama M, Ohseto F, Sasamoto K, Hamamoto T, Suzuki K, Watanabe M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal Commun. 1999; 36:47–50.

18. Yang JT, Wu CS, Martinez HM. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986; 130:208–269. PMID: 3773734.
19. Medzhitov R, Janeway CA Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002; 296:298–300. PMID: 11951031.

20. Yu XQ, Ma Y. Calcium is not required for immulectin-2 binding, but protects the protein from proteinase digestion. Insect Biochem Mol Biol. 2006; 36:505–516. PMID: 16731346.

21. Polotsky VY, Fischer W, Ezekowitz RA, Joiner KA. Interactions of human mannose-binding protein with lipoteichoic acids. Infect Immun. 1996; 64:380–383. PMID: 8557371.

22. Ip WK, Takahashi K, Moore KJ, Stuart LM, Ezekowitz RA. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J Exp Med. 2008; 205:169–181. PMID: 18180310.

23. Iwanaga S, Kawabata S. Evolution and phylogeny of defense molecules associated with innate immunity in horseshoe crab. Front Biosci. 1998; 3:D973–D984. PMID: 9727083.

24. Edge AS. Deglycosylation of glycoproteins with trifluoromethanesulphonic acid: elucidation of molecular structure and function. Biochem J. 2003; 376:339–350. PMID: 12974674.

25. Belogortseva N, Molchanova V, Glazunov V, Evtushenko E, Luk'yanov P. N-Acetyl-D-glucosamine-specific lectin from the ascidian Didemnum ternatanum. Biochim Biophys Acta. 1998; 1380:249–256. PMID: 9565695.
26. Banerjee S, Chaki S, Bhowal J, Chatterjee BP. Mucin binding mitogenic lectin from freshwater Indian gastropod Belamyia bengalensis: purification and molecular characterization. Arch Biochem Biophys. 2004; 421:125–134. PMID: 14678793.
27. Pereyra A, Zenteno R, Vázquez L, Martínez-Cairo S, Rodríguez A, Mendoza-Hernández G, Zenteno E, Agundis C. Characterization of lectin aggregates in the hemolymph of freshwater prawn Macrobrachium rosenbergii. Biochim Biophys Acta. 2004; 1673:122–130. PMID: 15279883.
28. Alpuche J, Pereyra A, Agundis C, Rosas C, Pascual C, Slomianny MC, Vázquez L, Zenteno E. Purification and characterization of a lectin from the white shrimp Litopenaeus setiferus (Crustacea decapoda) hemolymph. Biochim Biophys Acta. 2005; 1724:86–93. PMID: 15919156.

29. Gowda NM, Goswami U, Khan MI. Purification and characterization of a T-antigen specific lectin from the coelomic fluid of a marine invertebrate, sea cucumber (Holothuria scabra). Fish Shellfish Immunol. 2008; 24:450–458. PMID: 18282768.
30. Fabrick JA, Baker JE, Kanost MR. Innate immunity in a pyralid moth: functional evaluation of domains from a beta-1,3-glucan recognition protein. J Biol Chem. 2004; 279:26605–26611. PMID: 15084591.
31. Shi WX, Shen ZM, Sun C, Yang JT. Conformation and activity of Phaseolus coccineus var. rubronanus lectin. J Protein Chem. 1993; 12:153–157. PMID: 8489702.
32. Chandra NR, Prabu MM, Suguna K, Vijayan M. Structural similarity and functional diversity in proteins containing the legume lectin fold. Protein Eng. 2001; 14:857–866. PMID: 11742104.

33. Abdullaev FI, de Mejia EG. Antitumor effect of plant lectins. Nat Toxins. 1997; 5:157–163. PMID: 9407559.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download