Abstract
Interstitial cells of Cajal (ICCs) from the urinary bladder regulate detrusor smooth muscle activities. We cultured ICCs from the urinary bladder of mice and performed patch clamp and intracellular Ca2+ ([Ca2+]i) imaging to investigate whether cultured ICCs can be a valuable tool for cellular functional studies. The cultured ICCs displayed two types of spontaneous electrical activities which are similar to those recorded in intact bladder tissues. Spontaneous electrical activities of cultured ICCs were nifedipine-sensitive. Carbachol and ATP, both excitatory neurotransmitters in the urinary bladder, depolarized the membrane and increased the frequency of spike potentials. Carbachol increased [Ca2+]i oscillations and basal Ca2+ levels, which were blocked by atropine. These results suggest that cultured ICCs from the urinary bladder retain rhythmic phenotypes similar to the spontaneous electrical activities recorded from the intact urinary bladder. Therefore, we suggest that cultured ICCs from the urinary bladder may be useful for cellular and molecular studies of ICCs.
Interstitial cells of Cajal (ICCs) play an important role regulating smooth muscle cells by generating spontaneous pacemaker potentials and mediating signals from enteric neurons to smooth muscle cells as well as serving as stretch sensors in the gastrointestinal tract [1,2]. ICCs are distributed throughout the urinary tract and play an important role regulating smooth muscle activities [3,4]. Several types of ICCs have been found throughout the urinary bladder in several species [5,6]. ICCs are c-kit positive cells and spontaneous phasic contractions of the urinary bladder are inhibited by c-kit blocker [7]. The urinary bladder is innervated with cholinergic, peptidergic, and nitrergic nerve fibers, revealing close relationship with ICCs, and indicating that urinary bladder ICCs regulate smooth muscle activities and mediate neural signals to smooth muscles [6,8]. It has been reported that enhanced spontaneous phasic contractions in overactive bladder may be caused by release of acetylcholine (ACh) from cholinergic nerves acting on muscarinic receptors in ICCs [9]. ICCs have mechanical sensitivity; thus, they contribute to mechanosensitive conductance during urinary bladder regulation [10], and the distribution of ICCs is altered in urinary bladder outlet obstruction (BOD) [11]. These results suggest that ICCs play an important role in urinary bladder physiology and pathology. Therefore, ICC investigations might benefit the understanding of urinary bladder pathophysiology. Although the physiological roles of ICCs have been demonstrated, many studies have been morphological using immunohistochemistry and electrophysiology of intact tissues. However, because intact urinary bladder tissues contain other cells (smooth muscle cells and neuronal cells), it is difficult to identify the pure functional and physiological roles of ICCs alone. Cultured ICCs from the gastrointestinal tract have spontaneous pacemaker potentials similar to slow waves recorded in intact tissues; therefore, cultured ICCs have been used to elucidate the functional role of ICCs [12-14]. In the present study, we investigated whether cultured ICCs from urinary bladder can be of benefit to understand the functional physiological roles like gastrointestinal ICCs. Thus, we cultured urinary bladder ICCs from mice and recorded spontaneous electrical potentials.
All experiments were performed according to the Guiding Principles for the Care and Use of Animals approved by the Ethics Committee of Chosun University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize both the number of animals used and their suffering. Balb/C mice (8~13 days old) of either sex were anesthetized with ether and sacrificed by cervical dislocation. The urinary bladder was removed and opened. The luminal contents were washed away with Krebs-Ringer bicarbonate solution. The tissues were pinned to the base of a Sylgard dish and the mucosa was removed by sharp dissection. Small strips of bladder tissues were equilibrated in Ca2+-free Hank's solution containing 5.36 mM KCl, 125 mM NaCl, 0.34 mM NaOH, 0.44 mM Na2HCO3, 10 mM glucose, 2.9 mM sucrose, 11 mM HEPES for 30 min, and the cells were dispersed with an enzyme solution containing 1.3 mg/ml collagenase (Worthington Biochemical, Lakewood, NJ USA), 2 mg/ml bovine serum albumin (Sigma, St. Louis, MO USA), 2 mg/ml trypsin inhibitor (Sigma) and 0.27 mg/ml ATP. Cells were plated onto sterile glass coverslips coated with murine collagen (2.5 µg/ml, Falcon/BD, Franklin Lakes, NJ USA) in 35 mm culture dishes. The cells were then cultured at 37℃ in a 5% CO2 incubator in smooth muscle growth medium (Clonetics, San Diego, CA USA) supplemented with 2% antibiotic/antimycotic (Gibco, Grand Island, NY USA) and murine stem cell factor (5 ng/ml; Sigma). ICCs were identified immunologically with the use of a monoclonal antibody for kit protein (ACK2) labeled with Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA).
Cultured ICCs was fixed in acetone (20℃/5 min), and the preparations were washed for 60 min in phosphate-buffered saline (PBS; 0.01 M, pH 7.4). Cultured ICCs were then incubated in 10% goat serum containing 1% bovine serum albumin for 1 h at room temperature to reduce nonspecific antibody binding. To examine the ICCs, cultured ICC were incubated overnight at 4℃ with a rat monoclonal antibody raised against Kit protein (ACK2; 5 µg ml-1 in PBS; Gibco-BRL, Gaithersburg, MD, U.S.A). Immunoreactivity was detected using fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Vector Laboratories Burlingame, CA, USA, 1:100 in PBS, 1 h, at room temperature). Control cultured ICCs were prepared in a similar manner, but omitting ACK2 from the incubation medium. The cells were examined under a confocal laser scanning microscope (FV300, Olympus, Tokyo, Japan) at an excitation wave-length appropriate for FITC (488 nm).
The whole-cell configuration of the patch-clamp technique was used to record membrane potentials (current clamp) from cultured ICCs after 2~3 days of culture. Currents or potentials were amplified by Axopatch 1-D and 200B (Axon Instruments, Foster City, CA, USA). A command pulse was applied using an IBM-compatible personal computer and pClamp software (ver. 6.1; Axon Instruments). The data were filtered at 5 kHz and displayed on an oscilloscope and a computer monitor. Results were analyzed using pClamp and Graph Pad Prism (ver. 2.01) software. All experiments were performed at 30℃.
The cells were bathed in a solution containing 5 mM KCl, 135 mM NaCl, 2 mM CaCl2, 10 mM glucose, 1.2 mM MgCl2, and 10 mM HEPES, adjusted to pH 7.2 with Tris. The pipette solution contained 20 mM K-aspartate, 120 mM KCl, 5 mM MgCl2, 2.7 mM K2ATP, 0.1 mM Na2GTP, 2.5 mM creatine phosphate disodium, 5 mM HEPES, 0.1 mM EGTA, adjusted to pH 7.2 with Tris.
The drugs used were carbachol, methoctramine, glycopyrrolate, ATP, and nifedipine. All drugs were purchased from Sigma.
Changes in intracellular Ca2+ concentration ([Ca2+]i) were monitored using fluo-3/AM, which was initially dissolved in dimethyl sulfoxide and stored at -20℃. Cultured ICCs on coverslips (25 mm) were rinsed twice with a bath solution (5 mM: KCl, 135 mM NaCl, 2 mM CaCl2, 10 mM glucose, 1.2 mM MgCl2 and 10 mM HEPES, adjusted to pH 7.4 with Tris). The coverslips were then incubated in the bath solution containing 5 µM fluo-3 with 5% CO2 at 37℃ for 5 min, rinsed two more times in the bath solution, mounted on a perfusion chamber, and were scanned every 0.4 seconds with a Nikon Eclipse TE200 inverted microscope equipped with a Perkin-Elmer Ultraview confocal scanner and a Hamamatsu Orca ER 12-bit CCD camera (×200). Fluorescence was excited at a wavelength of 488 nm, and emitted light was observed at 515 nm. The temperature of the perfusion chamber containing the cultured ICCs was kept at 30℃ during scanning of the Ca2+ imaging. Variations in intracellular Ca2+ fluorescence emission intensity were expressed as F1/F0, where F0 is the intensity of the first image.
The morphology of cultured ICCs was c-kit positive stellate or spindle-shaped and they were connected to each other (Fig. 1). We performed the current clamp technique with cultured ICCs. The cultured ICCs exhibited two types of spontaneous electrical activities. One was individual generation of spontaneous spike potentials (Fig. 2A). The other was bursting generation of spontaneous spike potentials (Fig. 2B). The resting membrane potential was -41.5±2.7 mV (n=7), the frequency was -2.7±0.5 cycles/5 min (n=7), and the amplitude was 37.7±4.7 mV (n=7) for individual spontaneous spike potentials. The resting membrane potential for bursting spontaneous spike potentials was -42.7±-5.1 mV (n=8), the frequency was mean 7.5±1.5 cycles/5 min (n=8), and the amplitude was 52.5±6.3 mV (n=8). The summarized data are shown in Fig. 2C-E. The application of nifedipine (L-type Ca2+ channel blocker; 10-6 M) almost blocked the spontaneous electrical activities in both types (Fig. 3) (n=5 in individual type and n=4 in bursting type, respectively).
Exposure to carbachol (10-8 M), a selective muscarinic receptor agonist, produced depolarization of the resting membrane with an increase of the frequency of spontaneous electrical activities of the individual type (Fig. 4A). In the presence of carbachol, the resting membrane potential changed from -38.7±2.1 mV to -22.5±3.7 mV and frequencies was changed from 1.8±0.5 cycles/5 min to 6.1±1.3 cycles/5 min in the individual type (n=4). However, carbachol produced depolarization of the resting membrane in the bursting type with a decrease in the frequency of spontaneous electrical activities (Fig. 4B, n=4). In the presence of carbachol, the resting membrane potential changed from -37.3±3.2 mV to -10.7±4.7 mV and the frequencies changed from 9.1±1.1 cycles/5 min to 2.3±1.3 cycles/5 min in the bursting type (n=3). The carbachol-induced effects were blocked by atropine (a muscarinic receptor antagonist; 10-6 M) in both types (Fig. 4C and 4D), indicating that muscarinic receptors act on ICCs (Fig. 4C). We also investigated ATP effects on spontaneous electrical activities in cultured ICCs. ATP showed very similar effects as carbachol. Exposure to ATP (5×10-6 M) produced depolarization of the resting membrane with an increase in the frequency of spontaneous electrical activities in the bursting type, indicating that purinergic transmission occurs in bladder ICCs (Fig. 5A). The summarized data are shown in Fig. 5B and 5C.
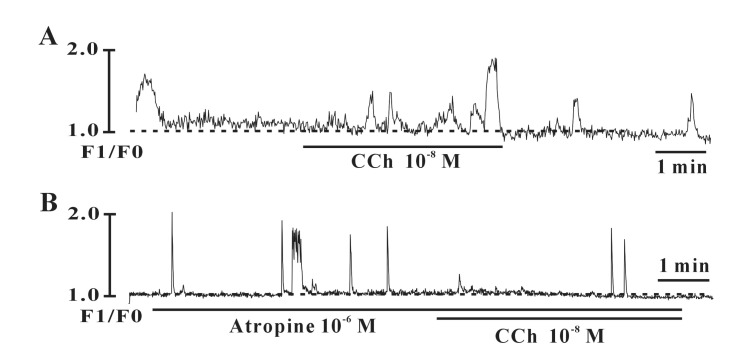
As intracellular Ca2+ ([Ca2+]i) oscillations in ICCs are the primary mechanism for pacemaking activity in the gastrointestinal tract, we examined the effects of carbachol on [Ca2+]i oscillations in ICCs. Spontaneous [Ca2+]i oscillations were observed in a time series from ICCs loaded with fluo3-AM. In the presence of carbachol (10-8 M), the [Ca2+]i oscillations increased and this effect was blocked by atropine (10-6 M) (Fig. 6, n=3).
We showed that the cultured ICCs from mouse urinary bladder exhibited spontaneous electrical activities which were similar to that those recorded from intact tissues. In addition, carbachol and ATP, both excitatory motor transmitters in the urinary bladder, depolarized the membrane and increased the frequency of spontaneous electrical activities.
ICCs contain several ion channels, receptors, and signal molecules, indicating that ICCs can be a therapeutic target in urinary bladder similar to the gastrointestinal tract [15-18]. It has been reported that ICCs populations increase in BOD, indicating that bladder ICCs play an important role in voiding [19]. Detrusor smooth muscles display spontaneous transient depolarization with mechanical contractions. In previous studies, detrusor smooth muscle showed two types of spontaneous electrical activities during intracellular microelectrode recording. Several detrusor smooth muscle cells show individually generated spontaneous single spike-shaped potentials, whereas other cells generate spontaneous bursting of several spike-shaped potentials [20,21]. In this study, cultured ICCs were c-kit positive stellate or spindle in shapes and formed networks with each others. The cultured ICCs also displayed two types of spontaneous electrical activity, individually generated spontaneous single spike-shaped potentials and bursting potentials which were similar to those recorded in intact urinary bladder tissues, suggesting that ICCs may act as pace-making cells in the urinary bladder. Spontaneous electrical activities in the intact urinary bladder are abolished by L-type Ca2+ channels blockers [22]. We found that the spontaneous electrical activities of cultured ICCs were blocked by nifedipine, suggesting that L-type Ca2+ channels are critical to generate the spontaneous electrical activity in urinary bladder ICCs. L-type Ca2+ channels have been recorded in isolated ICCs from the urinary bladder [23]. These results suggest that cultured ICCs retain the spontaneous electrical phenotype that similar to that recorded from intact tissues.
The urinary bladder is innervated by the autonomic nervous system. Parasympathetic innervations induce detrusor smooth muscle contraction with ACh after binding to muscarinic receptors [24]. ATP is also co-released with ACh from parasympathetic nerves and released from urothelial cells during bladder distension; thus, inducing contraction of detrusor muscles after binding to purinergic receptors [25,26]. In contrast, sympathetic innervation and nitric oxide induce relaxation via β-receptors and the cGMP pathway, respectively [27,28]. Bladder ICCs are innervated with cholinergic nerve fibers and the neurogenic-induced detrusor smooth muscle contractions are blocked by a c-kit blocker, indicating that bladder ICCs mediate neural signals to detrusor muscles [7]. ACh depolarizes the membrane and increases spontaneous phasic contractions of detrusor smooth muscles [21]. In the present study, carbachol, a muscarinic receptor agonist, depolarized the membrane and increased the frequency of spike potentials. Furthermore, the potentials were blocked by atropine, suggesting that cholinergic stimulation modulates detrusor smooth muscle contractions through ICCs after stimulation by muscarinic receptors. The depolarization by carbachol was prominent in spontaneous bursting type spike potentials, whereas increased frequency was prominent in the individual type of spike potentials. We do not know the differences in the effects of carbachol now. Several types of ICCs are distributed in urinary bladder. Therefore we think that this different effect by carbachol may be due to the ICC cell type. However, we should study further to identify the mechanism of muscarinic receptor stimulation.
ATP is another excitatory neurotransmitter that mediates atropine-resistant fast excitatory junction potentials via purinergic receptors in the urinary bladder [29]. ATP evokes a transient depolarization in the intact urinary bladder tissues [22]. In the present study, external ATP increased the frequency of spike potentials and depolarized the membrane in cultured ICCs, which was very similar to that those reported in intact urinary bladder tissues [29]. This means that ATP can modulates bladder activity through ICCs.
[Ca2+]i oscillations are essential to generate pacemaker potentials in small intestinal ICCs. Pacemaking in the gastrointestinal tract is initiated by release of Ca2+ from the endoplasmic reticulum, and is followed by reuptake of Ca2+ into mitochondria [12]. Urinary bladder ICCs are capable of generating spontaneous Ca2+ transients, suggesting that they might be essential for generating spontaneous excitation and exposure to carbachol produced Ca2+ waves [30]. Carbachol increases firing Ca2+ transients in dispersed urinary bladder ICCs. In the present study, carbachol increased the Ca2+ oscillations, and these cabachol-induced effects were blocked by atropine, suggesting that carbachol effects on [Ca2+]i are correlated with effects obtained from spontaneous electrical activities by carbachol.
In conclusion, our cultured ICCs showed similar spontaneous electrical activities to those recorded from intact urinary bladder preparations and they maintained the spontaneous electrical phenotype. Therefore, we think that use of cultured ICCs may be a beneficial method for cellular and molecular patholophysiological studies of the urinary bladder.
ACKNOWLEDGEMENTS
This study was supported by the grant from the Research Institute of Medical Sciences, Chonnam National University (2011-CURIMS-DR006).
References
1. Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998; 4:848–851. PMID: 9662380.

2. Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006; 68:307–343. PMID: 16460275.

3. Thornbury KD, Hollywood MA, McHale NG, Sergeant GP. Cajal beyond the gut: interstitial cells in the urinary system--towards general regulatory mechanisms of smooth muscle contractility? Acta Gastroenterol Belg. 2011; 74:536–542. PMID: 22319963.
4. McCloskey KD. Interstitial cells of Cajal in the urinary tract. Handb Exp Pharmacol. 2011; (202):233–254. PMID: 21290229.

5. Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol. 2005; 173:1385–1390. PMID: 15758810.

6. Lagou M, De Vente J, Kirkwood TB, Hedlund P, Andersson KE, Gillespie JI, Drake MJ. Location of interstitial cells and neurotransmitters in the mouse bladder. BJU Int. 2006; 97:1332–1337. PMID: 16686734.

7. Min Y, He P, Wang Q, Jin X, Song B, Li L. The effects of the c-kit blocker glivec on the contractile response of urinary bladder. J Surg Res. 2011; 171:e193–e199. PMID: 21962730.

8. Vahabi B, McKay NG, Lawson K, Sellers DJ. The role of c-kit-positive interstitial cells in mediating phasic contractions of bladder strips from streptozotocin-induced diabetic rats. BJU Int. 2011; 107:1480–1487. PMID: 20735390.

9. Grol S, Essers PB, van Koeveringe GA, Martinez-Martinez P, de Vente J, Gillespie JI. M(3) muscarinic receptor expression on suburothelial interstitial cells. BJU Int. 2009; 104:398–405. PMID: 19338557.
10. Wang Y, Fang Q, Lu Y, Song B, Li W, Li L. Effects of mechanical stretch on interstitial cells of Cajal in guinea pig bladder. J Surg Res. 2010; 164:e213–e219. PMID: 20828727.

11. Kubota Y, Hashitani H, Shirasawa N, Kojima Y, Sasaki S, Mabuchi Y, Soji T, Suzuki H, Kohri K. Altered distribution of interstitial cells in the guinea pig bladder following bladder outlet obstruction. Neurourol Urodyn. 2008; 27:330–340. PMID: 17724735.

12. Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000; 525 Pt 2:355–361. PMID: 10835039.

13. Nakayama S, Ohya S, Liu HN, Watanabe T, Furuzono S, Wang J, Nishizawa Y, Aoyama M, Murase N, Matsubara T, Ito Y, Imaizumi Y, Kajioka S. Sulphonylurea receptors differently modulate ICC pacemaker Ca2+ activity and smooth muscle contractility. J Cell Sci. 2005; 118:4163–4173. PMID: 16141235.
14. Choi S, Yeum CH, Kim YD, Park CG, Kim MY, Park JS, Jeong HS, Kim BJ, So I, Kim KW. Receptor tyrosine and MAP kinase are involved in effects of H2O2 on interstitial cells of Cajal in murine intestine. J Cell Mol Med. 2010; 14:257–266. PMID: 20414970.
15. Anderson UA, Carson C, McCloskey KD. KCNQ currents and their contribution to resting membrane potential and the excitability of interstitial cells of Cajal from the guinea pig bladder. J Urol. 2009; 182:330–336. PMID: 19450820.

16. Deng J, He P, Zhong X, Wang Q, Li L, Song B. Identification of T-type calcium channels in the interstitial cells of Cajal in rat bladder. Urology. 2012; 80:1389.e1. 1389.e7. PMID: 22995572.

17. McCloskey KD. Characterization of outward currents in interstitial cells from the guinea pig bladder. J Urol. 2005; 173:296–301. PMID: 15592100.

18. Shahi PK, Choi S, Zuo DC, Yeum CH, Yoon PJ, Lee J, Kim YD, Park CG, Kim MY, Shin HR, Oh HJ, Jun JY. 5-hydroxytryptamine generates tonic inward currents on pacemaker activity of interstitial cells of cajal from mouse small intestine. Korean J Physiol Pharmacol. 2011; 15:129–135. PMID: 21860590.

19. Kim SO, Oh BS, Chang IY, Song SH, Ahn K, Hwang EC, Oh KJ, Kwon D, Park K. Distribution of interstitial cells of Cajal and expression of nitric oxide synthase after experimental bladder outlet obstruction in a rat model of bladder overactivity. Neurourol Urodyn. 2011; 30:1639–1645. PMID: 21780165.

20. Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol. 2003; 140:146–158. PMID: 12967944.

21. Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004; 141:183–193. PMID: 14662721.

22. Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003; 140:159–169. PMID: 12967945.

23. McCloskey KD. Calcium currents in interstitial cells from the guinea-pig bladder. BJU Int. 2006; 97:1338–1343. PMID: 16686735.

24. Finney SM, Stewart LH, Gillespie JI. Cholinergic activation of phasic activity in the isolated bladder: possible evidence for M3- and M2-dependent components of a motor/sensory system. BJU Int. 2007; 100:668–678. PMID: 17627783.

25. O'Reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, McMahon SB. A quantitative analysis of purinoceptor expression in the bladders of patients with symptomatic outlet obstruction. BJU Int. 2001; 87:617–622. PMID: 11350400.
26. Burnstock G. Purinergic signalling in the lower urinary tract. Acta Physiol (Oxf). 2013; 207:40–52. PMID: 23176070.

27. Badawi JK, Seja T, Uecelehan H, Honeck P, Kwon ST, Bross S, Langbein S. Relaxation of human detrusor muscle by selective beta-2 and beta-3 agonists and endogenous catecholamines. Urology. 2007; 69:785–790. PMID: 17445682.

28. Yanai Y, Hashitani H, Hayase M, Sasaki S, Suzuki H, Kohri K. Role of nitric oxide/cyclic GMP pathway in regulating spontaneous excitations in detrusor smooth muscle of the guinea-pig bladder. Neurourol Urodyn. 2008; 27:446–453. PMID: 17929303.

29. Fujii K. Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. J Physiol. 1988; 404:39–52. PMID: 2908125.

30. McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002; 168:832–836. PMID: 12131376.

Fig. 1
Cultured ICCs from the mouse urinary bladder. The tunica muscularis of the urinary bladder was digested with collagenase, and the dispersed cells were cultured for 2 days. Confocal microscope image of Kit-immunopositive ICCs showed networks in culture.
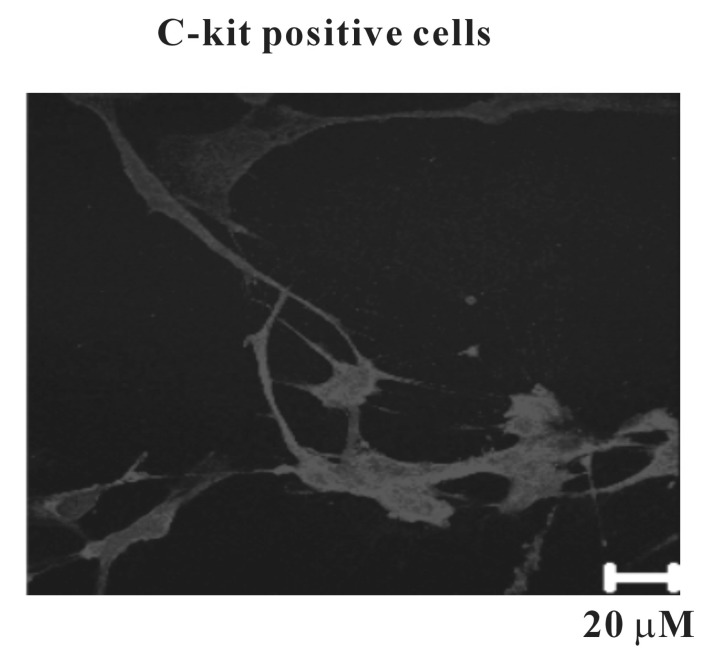
Fig. 2
Spontaneous electrical activities in cultured ICCs from mouse urinary bladder. The cultured ICCs exhibited two types' spontaneous electrical activities. One is individual generation of spontaneous spike potentials (A). The other is bursting generation of spontaneous spike potentials (B). Dotted lines indicate resting level of membrane potentials.
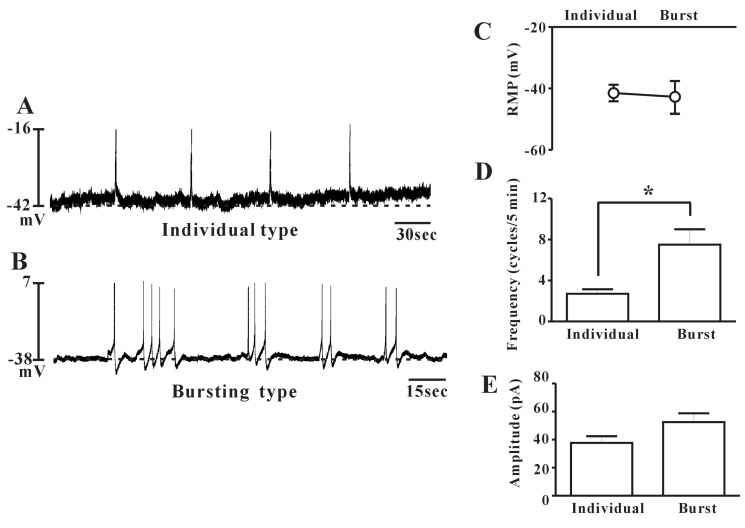
Fig. 3
Effect of nifedipine on spontaneous electrical activities in cultured ICCs from mouse urinary bladder. In cultured ICCs generated spontaneous spike potentials. Nifedipine (10-6 M) abolished the spontaneous spike potentials in both individual (A) and bursting (B) types. Dotted lines indicate resting level of membrane potentials.
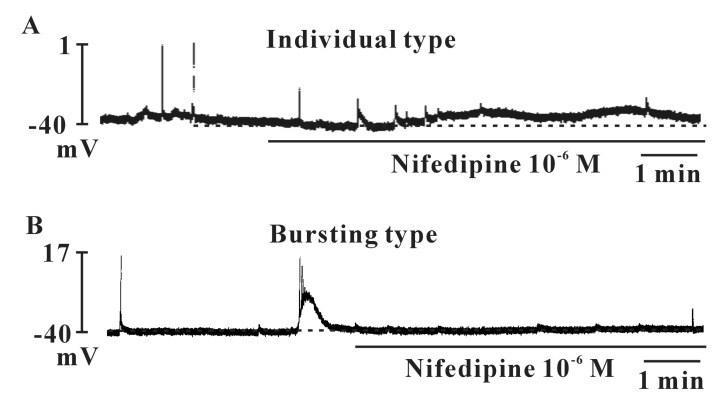
Fig. 4
Effects of carbachol spontaneous electrical activities in cultured ICCs from mouse urinary bladder. Carbachol (10-8 M) produced depolarization of the resting membrane with an increase of the frequency of spontaneous spike potentials in individual type (A). In the bursting type, carbachol produced depolarization of the resting membrane with a decrease of the frequency of spontaneous spike potentials (B). Atropine (10-6 M) blocked the carbachol-induced effects on spontaneous spike potentials in both individual (C) and bursting (D) types. Dotted lines indicate resting level of membrane potentials. CCh: carbachol.
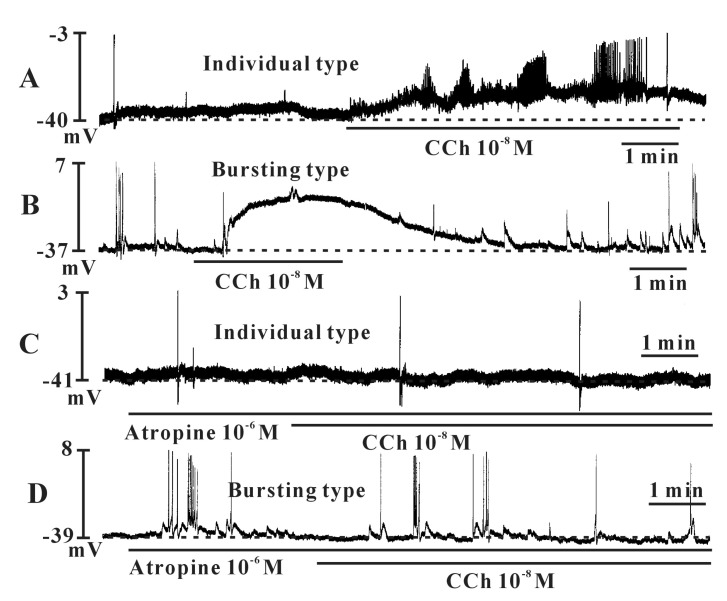
Fig. 5
Effects of ATP spontaneous electrical activities in cultured ICCs from mouse urinary bladder. ATP (5×10-6 M) produced depolarization of the resting membrane with an increase of the frequency of spontaneous spike potentials in bursting type (A). Dotted lines indicate resting level of membrane potentials. The changed values of resting membrane potential and frequency by ATP are summarized in (B) and (C).
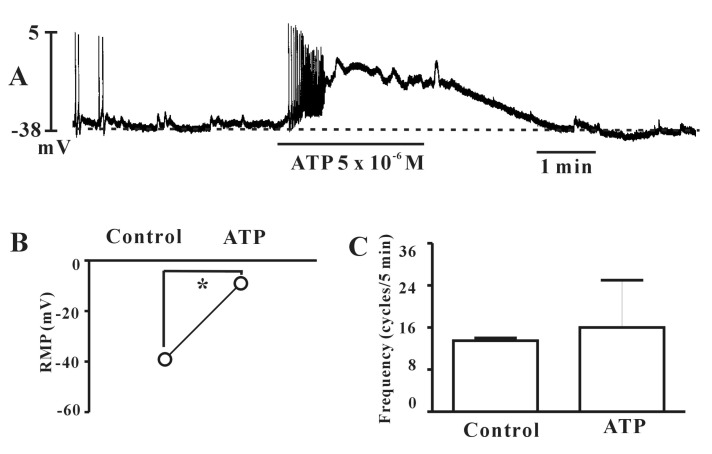
Fig. 6
The regulation of carbachol on spontaneous Ca2+ oscillation in cultured ICCs from the mouse urinary bladder. (A) Sequential fluorescence intensity images of Fluo-3 loaded cultured ICCs by carbachol (10-8 M). In the presence of carbachol, the [Ca2+]i oscillations were increased. (B) Sequential fluorescence intensity images of Fluo-3 loaded cultured ICCs by carbachol in the presence of atropine (10-6 M). Atropine blocked the carbachol-induced effects on [Ca2+]i oscillations. CCh, carbachol.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download