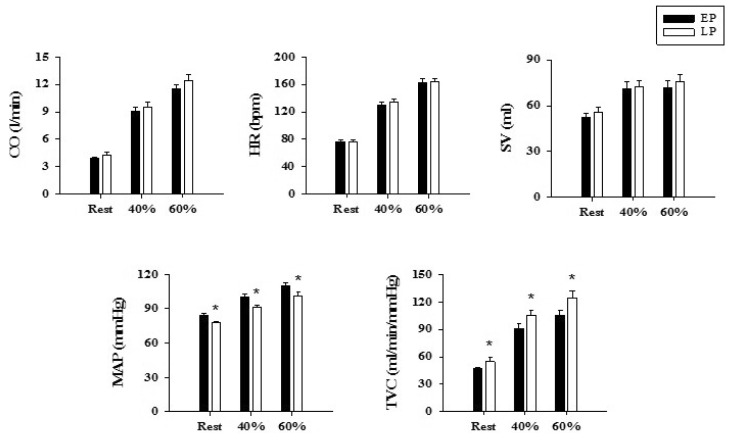
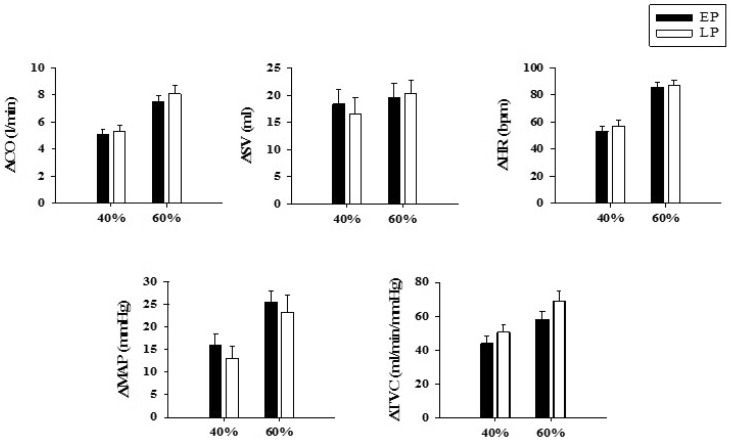
1. Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood). 2010; 235:111–118. PMID:
20404025.

2. Dunne FP, Barry DG, Ferriss JB, Grealy G, Murphy D. Changes in blood pressure during the normal menstrual cycle. Clin Sci (Lond). 1991; 81:515–518. PMID:
1657498.

3. Kavitha C, Jamuna BL, Vijayakumar GS. Pressor response during normal menstrual cycle. Int J Biol Med Res. 2012; 3:1975–1981.
4. Kelleher C, Joyce C, Kelly G, Ferriss JB. Blood pressure alters during the normal menstrual cycle. Br J Obstet Gynaecol. 1986; 93:523–526. PMID:
3707887.

5. Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab. 2001; 86:5389–5395. PMID:
11701712.

6. Schmitt PM, Kaufman MP. Estrogen attenuates the exercise pressor reflex in female cats. J Appl Physiol (1985). 2003; 95:1418–1424. PMID:
12819220.

7. Fadel PJ, Wang Z, Watanabe H, Arbique D, Vongpatanasin W, Thomas GD. Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J Physiol. 2004; 561:893–901. PMID:
15498809.

8. Kaplan V, Bucklar GB, Bloch KE. Noninvasive monitoring of cardiac output during exercise by inductance cardiography. Med Sci Sports Exerc. 2003; 35:747–752. PMID:
12750583.

9. Hietanen E. Cardiovascular responses to static exercise. Scand J Work Environ Health. 1984; 10:397–402. PMID:
6535242.

10. Kaufman MA, Forster HV. Reflexes controlling circulatory, ventilatory, and airway responses to exercise. Handbook of physiology Sect. 12. Exercise: regulation and integration of multiple systems. Bethesda: 1996.
11. Swift DL, Earnest CP, Katzmarzyk PT, Rankinen T, Blair SN, Church TS. The effect of different doses of aerobic exercise training on exercise blood pressure in overweight and obese postmenopausal women. Menopause. 2012; 19:503–509. PMID:
22547251.

12. Lewandowski J, Pruszczyk P, Elaffi M, Chodakowska J, Wocial B, Switalska H, Januszewicz W, Zukowska-Grojec Z. Blood pressure, plasma NPY and catecholamines during physical exercise in relation to menstrual cycle, ovariectomy, and estrogen replacement. Regul Pept. 1998; 75-76:239–245. PMID:
9802415.

13. Hartwich D, Aldred S, Fisher JP. Influence of menstrual cycle phase on muscle metaboreflex control of cardiac baroreflex sensitivity, heart rate and blood pressure in humans. Exp Physiol. 2013; 98:220–232. PMID:
22613743.

14. Kim A, Deo SH, Fisher JP, Fadel PJ. Effect of sex and ovarian hormones on carotid baroreflex resetting and function during dynamic exercise in humans. J Appl Physiol (1985). 2012; 112:1361–1371. PMID:
22267388.

15. Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR, Sinoway LI. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol (1985). 1998; 85:2075–2081. PMID:
9843528.
16. Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach. Philadelphia: Elsevier Saunders;2005.
17. English JL, Jacobs LO, Green G, Andrews TC. Effect of the menstrual cycle on endothelium-dependent vasodilation of the brachial artery in normal young women. Am J Cardiol. 1998; 82:256–258. PMID:
9678305.
18. Richard R, Lonsdorfer-Wolf E, Charloux A, Doutreleau S, Buchheit M, Oswald-Mammosser M, Lampert E, Mettauer B, Geny B, Lonsdorfer J. Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Eur J Appl Physiol. 2001; 85:202–207. PMID:
11560071.

19. Tordi N, Mourot L, Matusheski B, Hughson RL. Measurements of cardiac output during constant exercises: comparison of two non-invasive techniques. Int J Sports Med. 2004; 25:145–149. PMID:
14986199.

20. Bougault V, Lonsdorfer-Wolf E, Charloux A, Richard R, Geny B, Oswald-Mammosser M. Does thoracic bioimpedance accurately determine cardiac output in COPD patients during maximal or intermittent exercise? Chest. 2005; 127:1122–1131. PMID:
15821184.

21. Choi OH, Lee SH, Kim EJ, Kim KH, Rhim BY. Expression of nitric oxide synthase and endothelin-1 in human uterine artery from full-term pregnancies. Korean J Physiol Pharmacol. 2005; 9:165–172.
22. Havlik RJ, Phillips CL, Brock DB, Lohman K, Haskell W, Snell P, O'Toole M, Ribisl P, Vaitkevicius P, Spurgeon HA, Lakatta EG, Pullen P. Walking may be related to less vascular stiffness in the Activity Counseling Trial (ACT). Am Heart J. 2005; 150:270–275. PMID:
16086929.

23. Tanaka H, Safar ME. Influence of lifestyle modification on arterial stiffness and wave reflections. Am J Hypertens. 2005; 18:137–144. PMID:
15691628.

24. Wyss JM, Carlson SH. Effects of hormone replacement therapy on the sympathetic nervous system and blood pressure. Curr Hypertens Rep. 2003; 5:241–246. PMID:
12724057.

25. Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000; 101:862–868. PMID:
10694525.

26. Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension. 1996; 28:330–334. PMID:
8794812.

27. Westendorp IC, Bots ML, Grobbee DE, Reneman RS, Hoeks AP, Van Popele NM, Hofman A, Witteman JC. Menopausal status and distensibility of the common carotid artery. Arterioscler Thromb Vasc Biol. 1999; 19:713–717. PMID:
10073978.