Abstract
The aim of this study was to evaluate the synergistic potentiation effect of ineffective doses of dexmedetomidine on antinociception induced by morphine and fentanyl in acute pain model in rats. Seventy albino Wistar rats were separated into 7 groups. Data for the control and sham groups were recorded. The ineffective dose of dexmedetomidine was investigated and found to be 3 µ g/kg. Each group was administered the following medications: 3 mg/kg morphine (intraperitoneal) to Group 3, 5 µg/kg fentanyl (intraperitoneal) to Group 4, dexmedetomidine 3 µ g/kg (subcutaneously) to Group 5, dexmedetomidine 3 µg/kg (subcutaneous)+3 mg/kg morphine (intraperitoneal) to Group 6 and finally 3 µg/kg dexmedetomidine (subcutaneous)+5 µg/kg fentanyl (intraperitoneal) to Group 7. Just before the application and 15, 30, 60, 90 and 120 min after the administration of medication, two measurements of tail flick (TF) and hot plate (HP) tests were performed. The averages of the measurements were recorded. TF and HP latencies were the main outcomes. The analgesic effect of the combinations with dexmedetomidine+morphine (Group 6) and dexmedetomidine+fentanyl (Group 7), compared to the analgesic effect of morphine alone and fentanyl alone was significantly higher at 15, 30, 60 and 90 minutes after administration. In this study, dexmedetomidine in ineffective doses, when combined with morphine and fentanyl, potentiates the effects of both morphine and fentanyl.
Go to : 
Nowadays, the drugs used in treatment of pain can be divided into two pharmacological groups: non-opioids (adjuvants) and opioids [1-3]. Because the most common opioids used as analgesics have side effects like dependence and tolerance, as well as respiratory depression and constipation, while non-steroidal anti-inflammatory drugs (NSAID) have serious gastrointestinal side effects and also cause kidney damage [1,4,5], researchers are actively working to develop new adjuvant drugs. Therapeutic benefits of auxiliary drugs can be summarized as follows: they contribute to the formation of a strong analgesic as well as give the opportunity to reduce the dosage of major drugs; in particular, they reduce the frequency of opioid side effects such as nausea, vomiting, constipation, pruritus, sedation, and respiratory depression [6]. According to this, an alternative approach to mono-therapy that may be useful in the treatment of pain is to combine opioids with adjuvant analgesics or with other drugs that, on their own, do not have analgesic effectiveness but nevertheless amplify the effects of the opioid, thus requiring a smaller dose of the opioid and reducing its side effects [6].
Dexmedetomidine is a strong and highly selective α2-adrenoceptor agonist which has a wide spectrum of pharmacological properties. α2-adrenergic agonists provide sedation, anxiolysis, and hypnosis as well as analgesia and sympatholytic properties. In recent years, dexmedetomidine has attained an important place in researches, as well as in the treatment of pain, because it has analgesic effects comparable to opioids but does not show the side effects of opioids, like dependence and tolerance. This drug's effect mechanism, which is reported as acting through opioidergic, noradrenergic, and serotonergic receptors, is not completely clarified [7,8]. It has been widely accepted that co-administration of α2-adrenergic receptor agonists and µ receptor agonists produces synergistic antinociceptive effects in clinically effective doses. However, our study is a novel study that differs from other studies in that it uses ineffective doses of dexmedetomidine to achieve a potentiation effect.
In this study we aimed to evaluate the synergistic potentiation of dexmedetomidine in ineffective doses on antinociception induced by morphine and fentanyl in an acute pain model which we made through a rat tail-flick and hot plate method.
Go to : 
Adult male Wistar albino rats weighing 200~220 g (n=70) were used for the experiments. The animals were fed a standard laboratory diet and water ad libitum and were kept at 22±2℃ with a 12-h light/dark cycle. Animals were acclimatized to laboratory conditions before the test. All experiments were carried out blindly between 10:00 and 15:00 h (n=10 in each experimental group). Ethical approval for this study (No: 215) was provided by the Animal Ethical Committee of Cumhuriyet University on 30 September 2010.
Morphine sulfate (Cumhuriyet University Hospital, Turkey), fentanyl (Fentanyl citrate®, 50 mg/ml, Abbott, USA) and dexmedetomidine HCI (Precedex®, 200 µg/2 ml, Meditera, Turkey) were dissolved in saline. Drugs were prepared immediately prior to use; morphine and fentanyl were injected intraperitoneally (i.p.) and dexmedetomidine was injected subcutaneously (s.c.) in a volume of 10 ml/kg.
The nociceptive response to the tail-flick (TF) test is usually attributed to central mechanisms at the spinal level [9,10]. A standardized TF device (May TF 0703 Tail-flick Unit, Commat, Turkey) was used to evaluate thermal nociception. The radiant heat source was focused on the distal portion of the tail at 3 cm after administration of the vehicle or study drugs. Following vehicle or compound administration, tail-flick latencies (TFL) were obtained. The infrared intensity was adjusted so that basal TFL occurred at 2.8±0.4. Animals with a baseline TFL below 2.4 or above 3.2 s were excluded from further testing. The cutoff latency was set at 15 s to avoid tissue damage. Any animal not responding after 15 s was excluded from the study. Baseline measurements were performed twice before the administration of morphine (3 mg/kg), fentanyl (5 µg/kg), and dexmedetomidine (5 µg/kg), while and other measurements were performed twice at 15, 30, 60, 90, 120 min after administration and mean measurement values were recorded for each time point.
The response on the hot-plate (HP) is generally a result from a combination of central and peripheral mechanisms [10]. In this test, animals were individually placed on a hot plate (Eddy's Hot-Plate) with the temperature adjusted to 55±1℃. The latency to the first sign of paw licking or jump response to avoid the heat was taken as an index of the pain threshold; the cut-off time was 30 s in order to avoid damage to the rats' paws. HP tests were performed immediately after the tail flick test was completed.
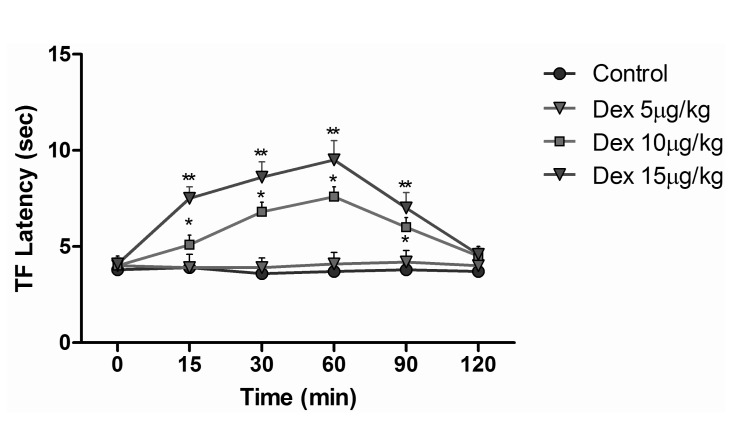
Animals were randomly divided 7 groups as control, sham, dexmedetomidine, morphine, fentanyl, dexmedetomidine+morphine, and dexmedetomidine+fentanyl. Animals in the control group were given no medication. Animals in the sham group were given 1 ml i.p. of saline. Dexmedetomidine group animals were injected 5 µg/kg s.c. dexmedetomidine, which was determined to be an ineffective stand-alone dose by the preliminary dose-response tests in our study (Fig. 1). Three mg/kg i.p. morphine was the dose we used for animals in the morphine group. Animals in the fentanyl group were given a 5 µg/kg i.p. dose that we indicated as an equivalent dose to morphine in our study. In the dexmedetomidine+morphine group, dexmedetomidine 5 µg/kg s.c. and morphine 3 mg/kg i.p. were used. Dexmedetomidine+fentanyl group animals were given dexmedetomidine 5 µg/kg s.c. and fentanyl 5 µg/kg i.p. TF and HP tests were performed at baseline (before drug administration) and at 15, 30, 60, 90 and 120 min after administration as a model of acute pain in rats (n=10 in each group). The rota rod performance test was used to evaluate the side effects of drugs by locomotor coordination of rats that received drugs or saline. The apparatus (COMMAT Ltd., Ankara, Turkey) consisted of three separate compartments and a rotating rod with a diameter of 7 cm. The rats were pre-trained on the rota rod apparatus for 3 days and then tested on the accelerating rod, in which the speed of the spindle was increased from 4 to 40 rpm over a period of 5 min. On the experiment day, each rat was tested for three times at 5 min intervals and the average of the responses was recorded as baseline values. Then the rats were injected with-depending on the group to which they were assigned-saline, dexmedetomidine 5 µg/kg, morphine 3 mg/kg, fentanyl 5 mg/kg, dexmedetomidine+morphine, or dexmedetomidine+fentanyl and tested at 15 and 30 min after injections. Results are expressed as the endurance time on the rota rod (Fig. 2) [11].
The results obtained are expressed as mean±SEM (standard error of mean). The effect of antinociception was measured. Groups were compared statistically using general linear models of two way ANOVA followed by Tukey test and t test when appropriate. p<0.05 was considered significant.
Go to : 
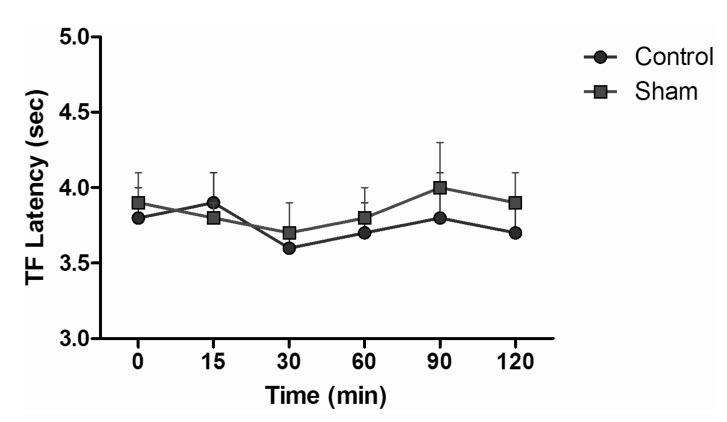
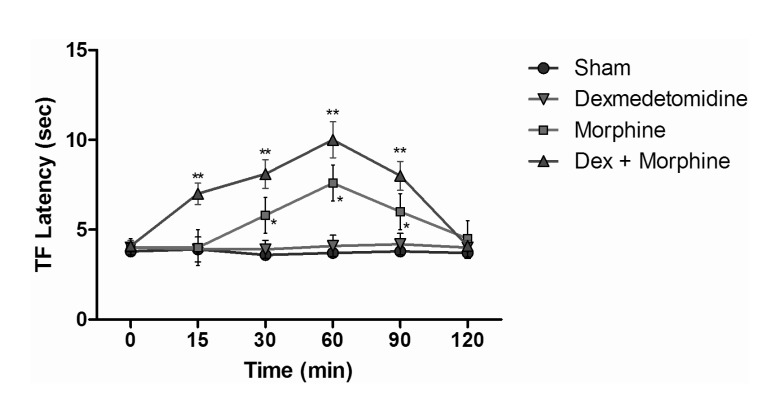
TF test results of intraperitoneal and subcutaneous administration of saline in the sham group showed no significant difference, when compared with control group values (Fig. 3), while the TF latencies of the dexmedetomidine group compared with the control group showed no significant difference statistically at any time. Morphine alone yielded a statistically significant analgesic effect starting from the 30 minute (min) after administration (p<0.05). TF latencies at the 60 min reached the maximum, and then gradually decreased and, at the 120 min, had decreased to the level of the control group. Application of morphine alone caused increased TF latencies that started at the 30 min, but the combination effect of the dexmedetomidine+morphine dosage started at the 15 min. Furthermore, when the TF latencies of the dexmedetomidine+morphine combination and the morphine-only groups were compared, the TF latencies of the combination were found to be significantly higher than the effect of morphine only at the 15, 30, 60 and 90 min (p<0.05) (Fig. 4). Fentanyl alone gave rise to a significant analgesic effect after the 15 min (p<0.05). This effect reached its maximum at the 60th min, then gradually decreased and, by the 90 min, had decreased to the level of the control group. The dexmedetomidine and fentanyl combination was parallel to fentanyl alone; it produced significant analgesia at the 15 min. This effect at the 60 min reached the maximum, and then gradually decreased until, by the 90 min, it had reached the level of the control group. TF latencies of the combination were statistically greater than fentanyl alone at 15, 30 and 60 min (p<0.05) (Fig. 5).
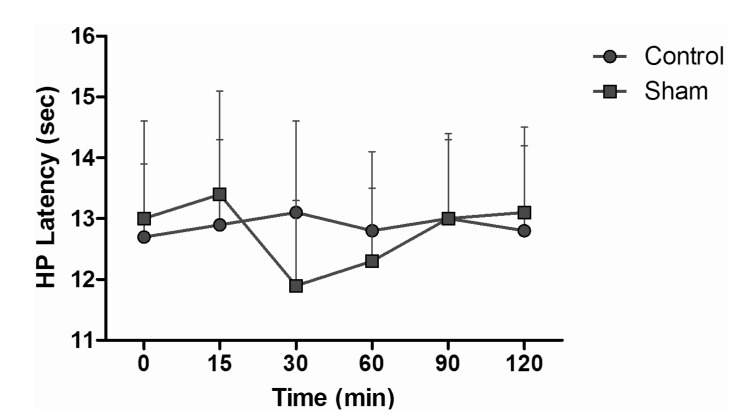
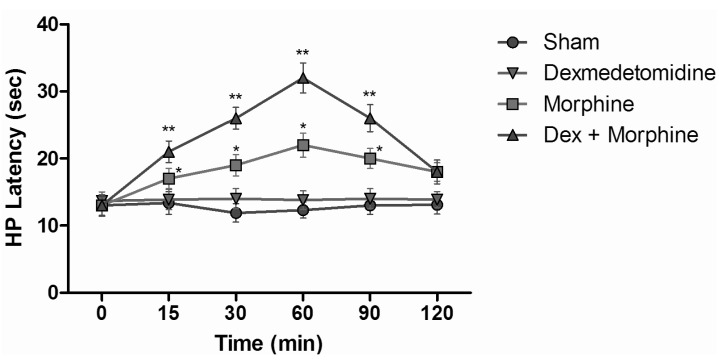
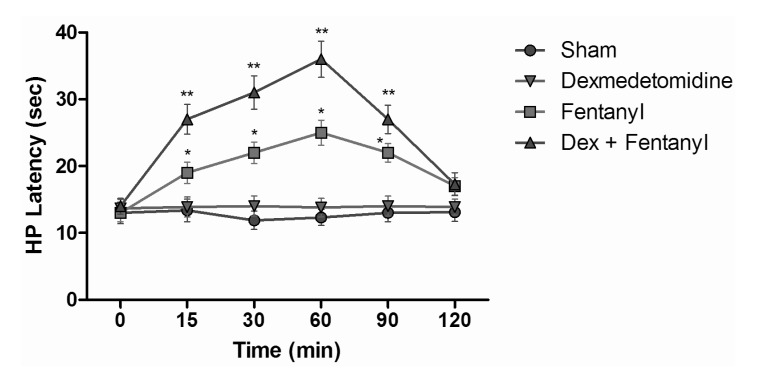
HP test results of intraperitoneal and subcutaneous administration of saline in the sham group showed no significant difference, when compared with control group values (Fig. 6). When HP latencies of the dexmedetomidine group were compared with the control group, no significant difference was found at any time. Both fentanyl and morphine provided significant analgesia starting from the 15 min; this effect reached its maximum level at the 60 min, and then it decreased. At the 120 min, although HP latencies decreased, they were not yet at control group levels (p<0.05). When the HP latency of the dexmedetomidine group was compared with the control group, a significant difference was not found at any time. Morphine induced significant analgesia that began at the 15 min. This effect at the 60 min reached its maximum and, after that, decreased. At the 120 min, although hot plate latencies had decreased, they were not yet at the levels of the control group. Dexmedetomidine+morphine combination provided significant analgesia starting at the 15 min and it was parallel to the application of fentanyl alone (p<0.05). This effect, at the 60 min, reached its maximum and, after that, decreased. Withdrawal latencies of the combination were statistically greater than morphine alone at the 15, 30, 60, and 90 min (p<0.05) (Fig. 7). Dexmedetomidine+fentanyl combination, from the 15 min, provided significant analgesia and it was parallel to the application of fentanyl alone. This effect reached its maximum level at the 60 min and it decreased gradually from that point. The withdrawal latencies of the combination were greater than the effect of fentanyl alone statistically significant at the 15, 30, 60, and 90 min (p<0.05) (Fig. 8).
Go to : 
We showed in our study that dexmedetomidine in ineffective doses had a potentiation effect on opioids. It has been well known that α2-adrenergic receptor agonists have a synergistic effect on opioids. However, our study is a novel study with an ineffective dose of dexmedetomidine and differs from other studies in that it shows the ability of ineffective doses of α2-adrenergic receptor agonists to increase the potentiality of opioids.
Despite the strong analgesic effects of opioid analgesics, their use is limited due to significant side effects such as respiratory depression and tolerance. Since opioids have such disadvantages and after research has shown that a variety of receptors other than opioid mechanisms are able to induce analgesia in the medulla spinalis, which is located in the central nervous system, the authors have been forced to research alternative drugs such as α2 receptor agonists. An electrophysiological study showed that α2-adrenoceptors and opiate receptors can interact in the modulation of nociceptive transmission in rat spinal cord [12]. It is well known that α2 receptor stimulation of the spinal cord plays a big role in the level of analgesia [8,13]. Paalzow [14] detected for the first time that systematic implementation of clonidine caused an analgesic effect; since then, this subject has led to several researches [15,16]. In these studies, an α2 receptor agonist from imidazole-class clonidine was used mostly due to its being easier to supply [17,18]. The selectivity of dexmedetomidine to α2 receptors (especially the α2A subtype) leads to more effective sedation and analgesia [19].
It is a known fact that α2-adrenoreceptor agonists utilize opioid receptors. Ossipov et al. [20] investigated the antinociceptive effects of morphine and subanalgesic doses of clonidine on rodents by applying the medication intrathecally. They found a significant potentiation in the TF test. Finally, they observed that the opioid effects returned after naloxone was injected. Their study proved that α2-adrenoreceptor agonists utilize opioid receptors.
In our study it was exposed through a TF test, which at spinal levels also potentiated the antinociceptive effects. However, it should not be forgotten that the tested α2 agonists were an ineffective stand-alone dose. Meert and De Kock [21] studied rat analgesic properties, specifically the potentiation effect of α2-adrenoreceptor agonists on opioids like fentanyl. They found that the α2 agonists that were tested may potentiate the analgesic effect of opioids, but had no real anti-nociceptive effect on spinal reflexes.
Slingsby et al. [22] studied the antinociceptive effects of dexmedetomidine with buprenorphine together and alone on cats. After all pharmaceutical applications, the cats tested with a thermal nociceptive threshold test. At the dose of 20 µg/kg im dexmedetomidine, a small analgesic effect was seen. Krzysztof et al. [23] created pain in rats with intraplantar formalin, administered morphine and morphine+clonidine to the rats, and then tested them by TF. In this study, the expected result was that the combination will be significantly more effective than morphine; Slingsby et al. saw the morphine+clonidine combination was more effective than morphine at low doses but not at high doses. In our study, the dexmedetomidine dose used was not an effective dose; however, when it had been combined with opioids, the analgesic effect was significantly potentiated.
There are several studies which used effective dose α2 agonists as a combination with opioids, and presented similar results. Puke and Wiesenfeld-Hallin [24] created experimental neuropathic pain in rats and then intrathecally administered morphine, morphine+clonidine, and morphine+clonidine+dexmedetomidine to the rats. For 21 days after drug administration, they looked for auto-amputation of toes and nails. They found that morphine alone was most effective in treating neuropathic pain, and that combination of morphine with α2 agonists was more useful and effective in treating chronic pain. Guneli et al. [25] examined the antinociceptive effects of tramadol and dexmedetomidine that were injected intraperitoneally to rats in whom acute and neuropathic pain had been generated. They found that the combination doses injected intraperitoneally were more effective than stand-alone doses and that HP and TF latencies were significantly higher. In this study they found that the antinociceptive synergistic effect of tramadol and low-dose dexmedetomidine was very strong and there was no sedation effect. However, the analgesic effect of the combination significantly increased. Gunes et al. [26] compared patient-controlled analgesia prepared with morphine+dexmedetomidine and prepared with morphine alone in patients who underwent a laminectomy. They fitted a morphine pump to the half of the 64 patients and a pump containing morphine+dexmedetomidine to the other half. Morphine consumption was reduced 30% in the combination group patients. They indicated that dexmedetomidine showed a synergistic effect with morphine.
In clinical practice, the analgesic effect of dexmedetomidine has been investigated in studies regarding the effect of reducing the use of opioids [8]. In healthy volunteers, a double-blind, placebo-controlled study was conducted in which ischemic chest pain was evaluated; it was seen that 0.25, 0.5 and 1 µg/kg IV dose of dexmedetomidine showed a similar effect with 2 µg/kg fentanyl [27]. Likewise, a single preoperative dose of dexmedetomidine reduced intraoperative and postoperative opioid analgesic requirements [27]. In a study on patients with mechanical ventilation after surgery that compared dexmedetomidine with placebo, it was found that dexmedetomidine decreased the requirement for morphine more than did the placebo [28]. In these clinical studies it was found that administration of perioperative dexmedetomidine helped to reduce both intraoperative and postoperative opioid and non-opioid analgesic requirements. Despite that, the sedative effect of dexmedetomidine restricts its use as a systemic analgesic [29].
Finally, we conclude that ineffective doses of dexmedetomidine, which is an α2 agonist, potentiate the antinociceptive effects of morphine and fentanyl when dexmedetomidine is combined with these opioids.
Go to : 
ABBREVIATIONS
NSAID
non-steroidal anti-inflammatory drugs
i.p
intraperitoneally
s.c
subcutaneously
TF
tail flick
TFL
tail flick latencies
HP
hot plate
Go to : 
References
1. Hernández L, Romero A, Almela P, García-Nogales P, Laorden ML, Puig MM. Tolerance to the antinociceptive effects of peripherally administered opioids. Expression of beta-arrestins. Brain Res. 2009; 1248:31–39. PMID: 19026993.
2. Ballesteros-Yanez I, Ambrosio E, Pérez J, Torres I, Miguéns M, García-Lecumberri C, DeFelipe J. Morphine self-administration effects on the structure of cortical pyramidal cells in addiction-resistant rats. Brain Res. 2008; 1230:61–72. PMID: 18657522.

3. Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. Effect of berberine on depression- and anxiety-like behaviors and activation of the noradrenergic system induced by development of morphine dependence in rats. Korean J Physiol Pharmacol. 2012; 16:379–386. PMID: 23269899.

4. Pergolizzi J, Böger RH, Budd K, Dahan A, Erdine S, Hans G, Kress HG, Langford R, Likar R, Raffa RB, Sacerdote P. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008; 8:287–313. PMID: 18503626.

5. Lanza FL. A guideline for the treatment and prevention of NSAID-induced ulcers. Members of the Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998; 93:2037–2046. PMID: 9820370.
6. Nader ND, Li CM, Dosluoglu HH, Ignatowski TA, Spengler RN. Adjuvant therapy with intrathecal clonidine improves postoperative pain in patients undergoing coronary artery bypass graft. Clin J Pain. 2009; 25:101–106. PMID: 19333153.

7. Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol. 2008; 21:457–461. PMID: 18660652.

9. Ramabadran K, Bansinath M, Turndorf H, Puig MM. The hyperalgesic effect of naloxone is attenuated in streptozotocin-diabetic mice. Psychopharmacology (Berl). 1989; 97:169–174. PMID: 2498924.

10. Kanaan SA, Saadé NE, Haddad JJ, Abdelnoor AM, Atweh SF, Jabbur SJ, Safieh-Garabedian B. Endotoxin-induced local inflammation and hyperalgesia in rats and mice: a new model for inflammatory pain. Pain. 1996; 66:373–379. PMID: 8880861.

11. Hamurtekin E, Gurun MS. The antinociceptive effects of centrally administered CDP-choline on acute pain models in rats: the involvement of cholinergic system. Brain Res. 2006; 1117:92–100. PMID: 16942753.

12. Sullivan AF, Dashwood MR, Dickenson AH. Alpha 2-adrenoceptor modulation of nociception in rat spinal cord: location, effects and interactions with morphine. Eur J Pharmacol. 1987; 138:169–177. PMID: 3040431.
13. Coursin DB, Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care. 2001; 7:221–226. PMID: 11571417.

14. Paalzow L. Analgesia produced by clonidine in mice and rats. J Pharm Pharmacol. 1974; 26:361–363. PMID: 4152777.

15. Dennis SG, Melzack R, Gutman S, Boucher F. Pain modulation by adrenergic agents and morphine as measured by three pain tests. Life Sci. 1980; 26:1247–1259. PMID: 7392798.

16. Chan SH, Lai YY. Effects of aging on pain responses and analgesic efficacy of morphine and clonidine in rats. Exp Neurol. 1982; 75:112–119. PMID: 7060671.

17. Pertovaara A, Hämäläinen MM, Kauppila T, Mecke E, Carlson S. Dissociation of the alpha 2-adrenergic antinociception from sedation following microinjection of medetomidine into the locus coeruleus in rats. Pain. 1994; 57:207–215. PMID: 7916451.
18. Pertovaara A. Antinociception induced by alpha-2-adrenoceptor agonists, with special emphasis on medetomidine studies. Prog Neurobiol. 1993; 40:691–709. PMID: 8097888.

19. Sabbe MB, Penning JP, Ozaki GT, Yaksh TL. Spinal and systemic action of the alpha 2 receptor agonist dexmedetomidine in dogs. Antinociception and carbon dioxide response. Anesthesiology. 1994; 80:1057–1072. PMID: 7912480.
20. Ossipov MH, Suarez LJ, Spaulding TC. Antinociceptive interactions between alpha 2-adrenergic and opiate agonists at the spinal level in rodents. Anesth Analg. 1989; 68:194–200. PMID: 2537587.
21. Meert TF, De Kock M. Potentiation of the analgesic properties of fentanyl-like opioids with alpha 2-adrenoceptor agonists in rats. Anesthesiology. 1994; 81:677–688. PMID: 7916547.
22. Slingsby LS, Murrell JC, Taylor PM. Combination of dexmedetomidine with buprenorphine enhances the antinociceptive effect to a thermal stimulus in the cat compared with either agent alone. Vet Anaesth Analg. 2010; 37:162–170. PMID: 20230567.

23. Przesmycki K, Dzieciuch JA, Czuczwar SJ, Kleinrok Z. Isobolographic analysis of interaction between intrathecal morphine and clonidine in the formalin test in rats. Eur J Pharmacol. 1997; 337:11–17. PMID: 9389375.

24. Puke MJ, Wiesenfeld-Hallin Z. The differential effects of morphine and the alpha 2-adrenoceptor agonists clonidine and dexmedetomidine on the prevention and treatment of experimental neuropathic pain. Anesth Analg. 1993; 77:104–109. PMID: 8100405.
25. Guneli E, Karabay Yavasoglu NU, Apaydin S, Uyar M, Uyar M. Analysis of the antinociceptive effect of systemic administration of tramadol and dexmedetomidine combination on rat models of acute and neuropathic pain. Pharmacol Biochem Behav. 2007; 88:9–17. PMID: 17651791.

26. Gunes Y, Ozbek TH, Gunduz HM, Gedik YE, Erman T, Ozcengiz D. Patient-controlled analgesia: comparison of morphine to dexmedetomidine plus morphine in patients undergoing laminectomy. Neurosurg Q. 2008; 18:178–181.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download