1. Nakamura K, Ozawa Y, Furuta Y, Miyazaki H. Effects of sodium polyacrylate (PANa) on acute esophagitis by gastric juice in rats. Jpn J Pharmacol. 1982; 32:445–456. PMID:
7109349.

2. Parmar NS, Hennings G. The gastric antisecretory activity of 3-methoxy-5,7,3'4'-tetrahydroxyflavan (ME)--a specific histidine decarboxylase inhibitor in rats. Agents Actions. 1984; 15:143–145. PMID:
6524513.

3. Alarcón de la Lastra C, Martín MJ, Motilva V. Antiulcer and gastroprotective effects of quercetin: a gross and histologic study. Pharmacology. 1994; 48:56–62. PMID:
8309988.

4. Haegele AD, Briggs SP, Thompson HJ. Antioxidant status and dietary lipid unsaturation modulate oxidative DNA damage. Free Radic Biol Med. 1994; 16:111–115. PMID:
8299986.
5. Okabe S, Takinami Y, Iwata K, Yanagawa T. Mucosal protective effect of leminoprazole on reflux esophagitis induced in rats. Jpn J Pharmacol. 1995; 69:317–323. PMID:
8786634.

6. Alvarez A, Pomar F, Sevilla , Montero MJ. Gastric antisecretory and antiulcer activities of an ethanolic extract of Bidens pilosa L. var. radiata Schult. Bip. J Ethnopharmacol. 1999; 67:333–340. PMID:
10617069.

7. Hollman PC, van Trijp JM, Buysman MN, van der Gaag MS, Mengelers MJ, de Vries JH, Katan MB. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997; 418:152–156. PMID:
9414116.

8. Biancani P, Billett G, Hillemeier C, Nissensohn M, Rhim BY, Szewczak S, Behar J. Acute experimental esophagitis impairs signal transduction in cat lower esophageal sphincter circular muscle. Gastroenterology. 1992; 103:1199–1206. PMID:
1327932.

9. Bell NJ, Hunt RH. Role of gastric acid suppression in the treatment of gastro-oesophageal reflux disease. Gut. 1992; 33:118–124. PMID:
1346769.

10. Biancani P, Sohn UD, Rich HG, Harnett KM, Behar J. Signal transduction pathways in esophageal and lower esophageal sphincter circular muscle. Am J Med. 1997; 103:23S–28S. PMID:
9422618.

11. Naya MJ, Pereboom D, Ortego J, Alda JO, Lanas A. Superoxide anions produced by inflammatory cells play an important part in the pathogenesis of acid and pepsin induced oesophagitis in rabbits. Gut. 1997; 40:175–181. PMID:
9071927.

12. Stein HJ, Hinder RA, Oosthuizen MM. Gastric mucosal injury caused by hemorrhagic shock and reperfusion: protective role of the antioxidant glutathione. Surgery. 1990; 108:467–473. PMID:
2382238.
13. Pihan G, Regillo C, Szabo S. Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 1987; 32:1395–1401. PMID:
3691277.

14. Kwak HS, Park SY, Nguyen TT, Kim CH, Lee JM, Suh JS, Whang WK, Sohn UD. Protective effect of extract from Rumex aquaticus herba on ethanol-induced gastric damage in rats. Pharmacology. 2012; 90:288–297. PMID:
23037147.

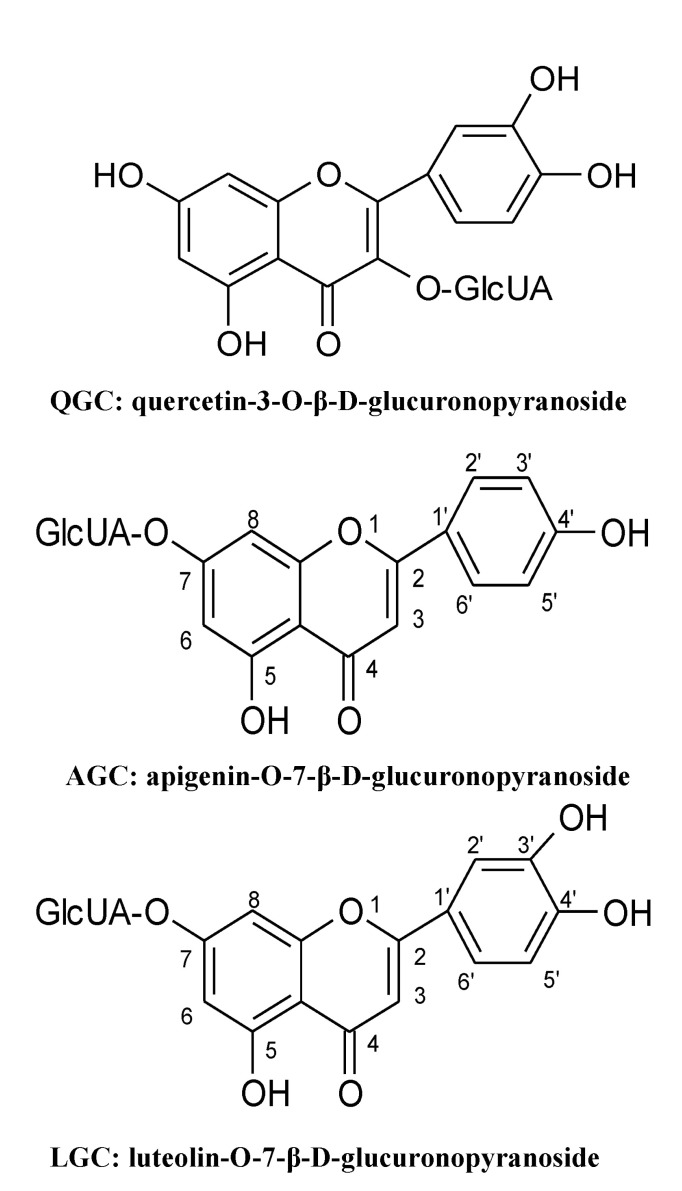
15. Min YS, Bai KL, Yim SH, Lee YJ, Song HJ, Kim JH, Ham I, Whang WK, Sohn UD. The effect of luteolin-7-O-beta-D-glucuronopyranoside on gastritis and esophagitis in rats. Arch Pharm Res. 2006; 29:484–489. PMID:
16833016.
16. Min YS, Lee SE, Hong ST, Kim HS, Choi BC, Sim SS, Whang WK, Sohn UD. The Inhibitory Effect of Quercetin-3-O-beta-DGlucuronopyranoside on Gastritis and Reflux Esophagitis in Rats. Korean J Physiol Pharmacol. 2009; 13:295–300. PMID:
19885013.
17. Min YS, Yim SH, Bai KL, Choi HJ, Jeong JH, Song HJ, Park SY, Ham I, Whang WK, Sohn UD. The effects of apigenin-7-O-beta-D-glucuronopyranoside on reflux oesophagitis and gastritis in rats. Auton Autacoid Pharmacol. 2005; 25:85–91. PMID:
15955027.

18. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963; 61:882–888. PMID:
13967893.
19. Grisham MB, Benoit JN, Granger DN. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990; 186:729–742. PMID:
2172726.
20. Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastrooesophageal reflux disease. Digestion. 1992; 51(Suppl 1):59–67. PMID:
1397746.

21. Ito M, Suzuki Y, Ishihara M, Suzuki Y. Anti-ulcer effects of antioxidants: effect of probucol. Eur J Pharmacol. 1998; 354:189–196. PMID:
9754920.

22. Murakami S, Muramatsu M, Otomo S. Inhibition of gastric H+, K(+)-ATPase by quercetin. J Enzyme Inhib. 1992; 5:293–298. PMID:
1285250.
23. Parmar NS, Hennings G. The gastric antisecretory activity of 3-methoxy-5,7,3'4'-tetrahydroxyflavan (ME)--a specific histidine decarboxylase inhibitor in rats. Agents Actions. 1984; 15:143–145. PMID:
6524513.

24. Gambhir SS, Goel RK, Das Gupta G. Anti-inflammatory & antiulcerogenic activity of amentoflavone. Indian J Med Res. 1987; 85:689–693. PMID:
2824354.
25. Macchia M, Barontini S, Bertini S, Di Bussolo V, Fogli S, Giovannetti E, Grossi E, Minutolo F, Danesi R. Design, synthesis, and characterization of the antitumor activity of novel ceramide analogues. J Med Chem. 2001; 44:3994–4000. PMID:
11689086.

26. Naito Y, Yoshikawa T, Yoshida N, Kondo M. Role of oxygen radical and lipid peroxidation in indomethacin-induced gastric mucosal injury. Dig Dis Sci. 1998; 43(9 Suppl):30S–34S. PMID:
9753223.
27. Tanaka J, Yuda Y. Lipid peroxidation in gastric mucosal lesions induced by indomethacin in rat. Biol Pharm Bull. 1996; 19:716–720. PMID:
8741581.

28. Fensome AC, Rodrigues-Lima F, Josephs M, Paterson HF, Katan M. A neutral magnesium-dependent sphingomyelinase isoform associated with intracellular membranes and reversibly inhibited by reactive oxygen species. J Biol Chem. 2000; 275:1128–1136. PMID:
10625655.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download