Abstract
We studied the sex different nicotine effect on evoked population spike amplitudes (ePSA) and connexin (Cx) expression in the hippocampus CA1 area of gerbils. Acute doses of nicotine bitartrate (0.5 mg/kg: NT-0.5) slightly reduced ePSA in males but markedly augmented that in females. Acute NT (5.0 mg/kg) markedly increased the ePSA in all gerbils. Unlike acute NT-0.5, repeated NT-0.5 injection (twice a day for 7 days) significantly increased the ePSA in males and slightly affected the NT-0.5 effect in females. The Cx36 and Cx43 expression levels as well as Cx expressing neuronal populations were significantly increased by repeated NT-0.5 in in both male and female gerbils, and particularly, Cx43 expression was somewhat prominent in females. These results demonstrated a sex difference with respect to the nicotine effect on hippocampal bisynaptic excitability, irrelevant to connexin expression.
Nicotine addiction is mainly attributable to activity of the mesolimbic dopaminergic system [1,2]. With respect to learning and memory, nicotine is known to enhance the fast synaptic current in the hippocampus by activating nicotinic acetylchoiline receptors (nAChR) on the presynaptic terminals of glutamatergic neurons [3] and also to elicit an increase in the population spike amplitude in the CA1 region [4,5].
On the other hand, various patterned functions of neural circuitries, for example generating circadian rhythms, function through the use of chemical synapses and electrical gap junctions [6]. Although gap junctional coupling is often assumed to be static, its plasticity has a role in neural activity [7]. Nicotine has been shown to activate spontaneous or electrically multicellular [Ca2+] transients via gap junction-delineated routes in neuronal cells [3]. On the contrary, nicotine was able to reduce the expression of gap junction proteins and the function of gap junctions in human umbilical vein endothelial cells [8].
According to a recent report [9], the amplitude of the population spikes recorded in the dentate after perforant path stimulation is potentiated by pretreatment with nicotine (0.5 mg/kg).
In this context, we hypothesize that sex difference with respect to the effect of nicotine on the amplitude of evoked population spike in the hippocampus CA1 region may result from the different expression levels of gap junctional proteins, such as neuron-specific connexin (Cx) 36 and nonspecific Cx43, respectively [10]. To address this, we measured the evoked population spike amplitude (ePSA) in the hippocampus CA1 region, induced by electrically stimulating the dentate gyrus, using male and female gerbils exposed to both acute and repeated nicotine treatments.
(-)-Nicotine hydrogen tartrate (Sigma Chemical Co., USA) was dissolved in sterile saline. Mongolian gerbils (50~70 g, Orient Bio Inc., Korea) were housed under controlled conditions (12:12 h light-dark cycle; 21~23℃; 55~65% humidity) for at least 7 days with free access to chow and water prior to their use in the experiment. The present study received approval from the Institutional Animal Care and Use Committee of Korea University College of Medicine.
Male and female gerbils were divided into 4 groups according to the nicotine treatment regimen. All groups were subcutaneously pretreated with saline (1.0 mg/kg) or nicotine ((-)-nicotine bitartrate, 0.5 mg/kg: NT-0.5) twice a day for 7 days and then exposed to a saline or NT-0.5 challenge (i.p. injection), respectively, on the 4th day after the last injection of the pretreatment. The groups were as follows: SS group: saline pretreatment followed by a saline challenge; SN group: saline pretreatment followed by a NT-0.5 challenge; NS group: NT-0.5 pretreatment followed by a saline challenge; NN group: NT-0.5 pretreatment followed by a NT-0.5 challenge.
Evoked population spikes were recorded according to modification to a previously described method [11,12]. Animals were placed in a stereotaxic apparatus (David Kopf, USA) under urethane anesthesia (1.0 g/kg, i.p. injection). Rectal temperatures were maintained at 37±0.5℃ using a thermostatic blanket and rectal thermometer.
A recording tungsten electrode (127 µm OD, 1 µm tip, 5.0 MΩ impedance, WPI, USA) was placed in the right hippocampal CA1 region (AP -1.5~-1.8 mm, lateral 1.0~1.5 mm, depth 1.2~1.5 mm). A bipolar stimulating electrode (127 µm OD, WPI, USA) was positioned in the ipsilateral dentate gyrus (AP -2.6~-3.0 mm, lateral 2.5~3.0 mm, depth 2.2~2.6 mm) and equipped with a rectangular electric stimulator to induce bisynaptic activation of the CA1 pyramidal cells by rectangular electrical stimuli (0.1 ms 0.2~0.5 Hz, 7~9 V; 1.5 x threshold voltage) (Fig. 1A).
The ePSA appeared at the latent time of 12±1.0 msec after electrical stimulation. It was estimated as the voltage from the midpoint between the two shoulders of the first and second positive waves to the peak of maximal negativity (Fig. 1B). To measure the ePSA corresponding to the extracellular field potential evoked by an afferent volley, the recording electrode was connected to a field effect transistor. The outputs were amplified, filtered (100 Hz~3 KHz) and digitized using an analog-to-digital converter (DigiData 1200B, Axon Instrument, USA) and then were stored on a personal computer.
At 1 hr after the last saline or NT-0.5 challenge, animals were deeply anesthetized with sodium pentobarbital (80mg/kg, i.p.) and transcardially perfused, with 0.1M phosphate buffer (PB), pH 7.4 containing 0.9% NaCl (PBS) and then with 0.1M PBS, pH 7.4 containing 4% freshly depolymerized paraformaldehyde (PFA). The brains were removed and submerged in cold PFA for 18 hrs and then were stored in 0.1 M PBS, pH 7.4 containing 30% sucrose solution at 4℃ for 48 hrs. After being frozen in dry ice, coronal sections (20 µm) were prepared (including -0.9~1.6 mm posterior to the bregma) using a Cryostat (Microm, GmbH, Germany), and were stored in cryoprotectant solution (0.1M PB, pH 7.4, containing 30% sucrose, 30% ethylene glycol, 1% polyvinylpyrrolidone).
At room temperature, the sections were treated with blocking solution (0.1M PBS, pH 7.4, containing 0.1% Triton X-100 and 2% normal bovine serum) for 2 hrs, and were then rinsed for 2×15 min with 0.1 M PBS (pH 7.4). Sections were then incubated at 4℃ overnight with 1:1 mixture of mouse monoclonal anti-mouse NeuN antibody (1:200, Chemicon Inc., USA) and either rabbit polyclonal anti-Cx36 antibody (1:500, Zymed Inc., USA) or rabbit polyclonal anti-Cx43 antibody (1:500, Zymed Inc., USA). Negative control sections were incubated without these primary antibodies. Sections were washed for 3×15 min with 0.1 M PBS (pH 7.4) and then were incubated with 1:1 mixture of donkey Cy3-conjugated anti-rabbit IgG (1:200, Chemicon Inc., USA) and donkey FITC-conjugated antimouse IgG (1:200, Chemicon Inc., USA) at room temperature for 2 hrs. Finally, sections were washed for 2×15 min with 0.1 M PB, pH 7.4, mounted on glass slides, and cover-slipped with anti-fade aqueous mounting medium (Biomeda Corp., USA).
Using an Olympus confocal microscope (Olympus FV300, Japan), fluorescence signals were captured with 1 µm interval through the z-axis of the section, covering 15 um in depth as 16 serial scans. The images were analyzed using MetaMorph software v. 7.0 (MDS Inc., USA). Anti-Cx36 and anti-Cx43 immunoreactive (IR) dots expressed on the anti-NueN positive neurons were quantified in the pyramidal layer of the dorsal hippocampus CA1 area, of which the boundaries were demarcated on the basis of their morphology and location.
To investigate whether acute and repeated nicotine administration effects on the excitability of hippocampal pyramidal neurons show any sex-related differences and nicotine-induced neuronal sensitization, we measured the ePSA in the pyramidal layer of the hippocampus CA1 area, evoked by electrical stimulation of the dentate gyrus in male and female gerbils.
In male gerbils, NT-0.5 decreased the ePSA by an average of 10.6±3.0%; however, NT-5.0 significantly increased the ePSA by an average of 31.0±2.5% during the recording periods (drug-time: F(12,108)=4.8, p=0.001).
On the contrary, both NT-0.5 and NT-5.0 markedly increased the ePSA by an average of 30.5±4.4% and 38.9±2.3%, respectively, in females (drug-time: F(12,102)=10.4, p=0.001) without any significant differences between NT-0.5 and NT-5.0 groups, showing a sex difference in response to acute nicotine dose (Fig. 2A).
Pretreatment with repeated NT-0.5 (s.c. injection, twice/d) for 7 and 28 days following NT-0.5 challenge significantly increased the ePSA in males (16.6±3.3 and 11.2±2.5%, respectively) (drug-time: F(12,108)=2.7, p=0.004) and also in females (37.2±3.0 and 36.9±4.2%, respectively) (drug-time: F(12,102)=5.8, p=0.001), showing that the repeated nicotineinduced sensitization of the synaptic excitability of the pyramidal neurons might be induced in male gerbils but not in females (Fig. 2B).
To address whether the hippocampal Cx36 and Cx43 expression was associated with sex-related differences in the acute nicotine effect on the ePSA and repeated nicotine-induced sensitization of the synaptic excitability of the hippocampal pyramidal neurons, we examined acute and repeated nicotine effects on the Cx36 and Cx43 expressions in the pyramidal layer of the hippocampus CA1 area (Fig. 3, 4).
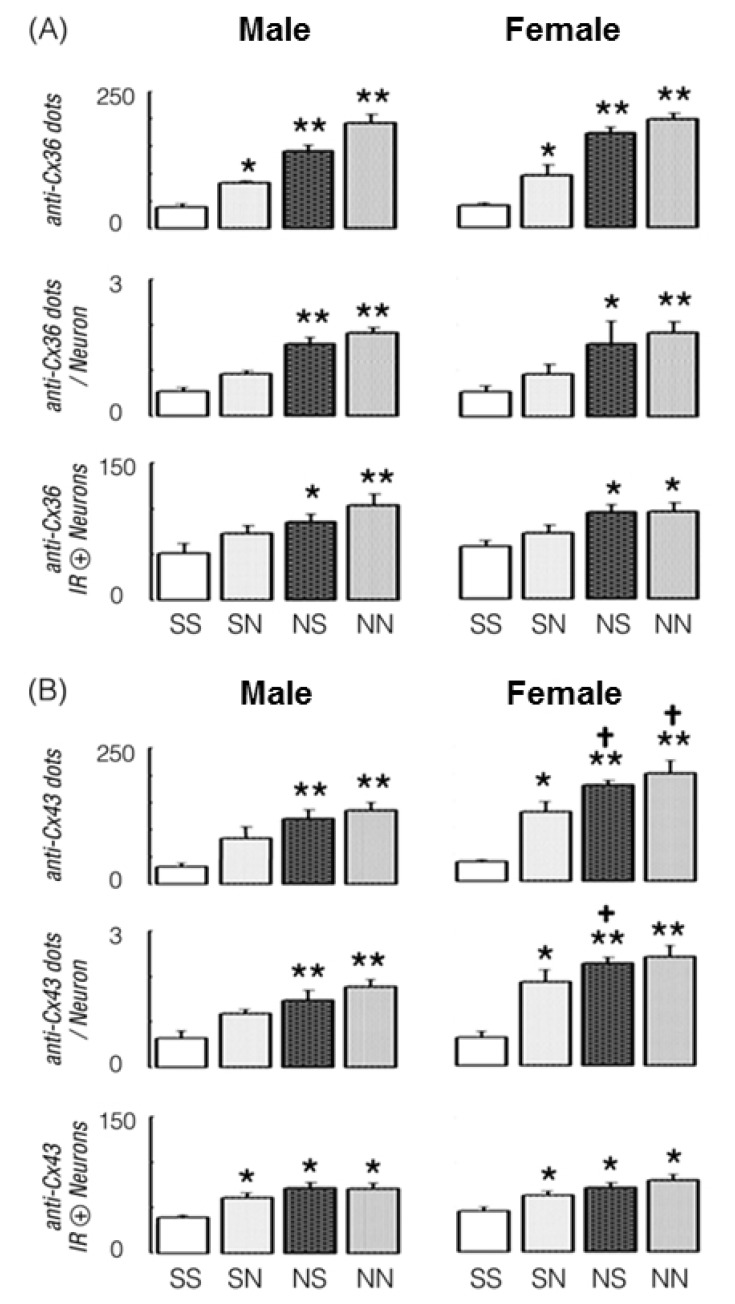
In male and female gerbils pretreated with repeated saline (s.c. injection, twice/d) for 7 days, the average number of anti-Cx36 IR dots in a pyramidal neuron and the number of anti-Cx36 IR positive neurons were moderately increased by NT-0.5 challenge, and so, the total number of anti-Cx36 IR dots in a pyramidal layer was significantly increased by acute NT-0.5 challenge in the males (114.8%, p=0.011) and females (142.7%, p=0.017).
On the other hand, in male and female gerbils pretreated with repeated NT-0.5 (s.c. injection, twice/d) for 7 days, the total number of anti-Cx36 IR dots was significantly increased by saline and NT-0.5 challenge in males (268.6%, p=0.001 and 401.0%, p=0.001) and females (341.9%, p=0.002 and 406.5%, p=0.001), which resulted from significant increases in the numbers of anti-Cx36 IR dots per neuron and anti-Cx36 IR positive neurons in both groups (Fig. 4A).
In male gerbils pretreated with repeated saline (s.c. injection, twice/d) for 7 days, the total number of anti-Cx43 IR dots and the average number of anti-Cx43 IR dots per neuron were moderately increased; however, the number of anti-Cx43 IR positive neurons was increased by NT-0.5 challenge (66.8%, p=0.01) (Fig. 4B).
In female gerbils pretreated with the same dosage regimen, the total number of anti-Cx43 IR dots was significantly increased by NT-0.5 challenge (263.8%, p=0.003), which resulted from increases in the average number of anti-Cx43 IR dots per neuron and the number of anti-Cx43 IR positive neurons. Furthermore, in male and female gerbils pretreated with repeated NT-0.5 (s.c. injection, twice/d) for 7 days, the total number of anti-Cx43 IR dots was significantly increased by saline and NT-0.5 challenge in males (280.1%, p=0.002 and 328.8%, p=0.001) and females (401.4%, p=0.001 and 467.6%, p=0.002), which resulted from significant increases in the average anti-Cx43 IR dots per neuron and the number of anti-Cx43 IR positive neurons (Fig. 4B).
These results suggest that acute and repeated nicotine administration can increase Cx36 and Cx43 expression in the hippocampus CA1 neurons, with a greater nicotine effect on Cx43 expression in females.
Estradiol is known to enhance long term potentiation (LTP) by glutamatergic synapses in the hippocampus CA1 area [13,14], as well as increasing gap junctions in the hypothalamus [15]. Like estrogens, androgens supplemented to both male and female gonadectomized rats increased dendritic spine density in the hippocampus CA1 area [16,17] and gap junctions of spinal motor neurons [18]. Therefore, we initiated this study to investigate whether there was any significant sexual dimorphism with respect to the acute nicotine effect on the ePSA and the repeated nicotine-induced augmentation of the ePSA in the pyramidal layer of the hippocampus CA1 region.
Acute NT-0.5 (about 0.17 mg/kg, as nicotine base) slightly reduced the ePSA in male gerbils but markedly augmented it in females. On the other hand, NT-5.0 (about 1.7 mg/kg, as nicotine base) markedly increased the ePSA in both male and female gerbils (Fig. 2A), showing a greater neuronal sensitivity of females to nicotine. But on the 4th day after the last dose of repeated NT-0.5 pretreatment (twice a day for 7 days or for 28 days), NT-0.5 challenge augmented the ePSA in males, but in female gerbil, the augmentation of ePSA by acute NT-0.5 was slightly altered by repeated NT-0.5 pretreatment (Fig. 2B), suggesting that repeated nicotine treatment would enhance the synaptic neuronal excitability of the pyramidal layer in males, unlike that in females.
These results are consistent with the previous reports that in vivo long-term potentiation in the hippocampal complex was induced by nicotine [3,19] and also enhanced by estrogens [13,14]. These results also suggest that androgens may reduce synaptic plasticity in the hippocampal CA1 region [20], opposing the reports that androgens increased synaptic density in the CA1 [16,17]. This disagreement may be ascribed to species differences (Sprague Dawley rat vs M. gerbil) or differences with respect to experimental design (morphological synapse density vs ePSA). In a recent study in our lab (unpublished data), however, acute NT-0.5 markedly increased the ePSA in castrated male gerbils, although reduced the ePSA in intact males.
Axonal coupling between the pyramidal neurons supports fast network oscillations in the hippocampus, which would be modulated by the dendritic coupling between interneuron dendrities, and these effects are supposed to require gap junctions (about 1.5 to 3 per neuron) [4]. Multicellular [Ca2+] transients of chromaffin cells were activated by nicotine, and their nicotine-induced interneuronal communication was blocked by carbenoxolone, a gap junction blocker [7].
Therefore, the second part of this study was focused on addressing whether there was any sex-related difference with respect to the acute nicotine or repeated nicotine effects on neuron-specific Cx36 and -nonspecific Cx43 levels in the pyramidal layer of the CA1 area. In male and female gerbils pretreated with saline or NT-0.5 twice a day for 7 days, NT-0.5 challenge on the 4th day after 3 days of withdrawal periods significantly increased the anti-Cx36 and anti-Cx43 IR dots in the pyramidal layer of the hippocampal CA1 area (Fig. 4). The effect of NT-0.5 challenge was markedly greater in the NT-0.5 pretreated gerbils than in those of saline pretreated gerbils, suggesting that repeated nicotine treatment would sensitize the connexin expression in the pyramidal neurons. In addition, the NT-0.5 induced increase of anti-Cx43 dots was significantly greater in females than in males, without any difference between sexes in number of anti-Cx43 IR positive neurons.
Considering that long-term plasticity of gap junctional coupling is controlled at the transcriptional level and by interactions with scaffolding proteins [7,21,22], our results seem to be a significant evidence for further study on the mechanism of nicotine-induced neuronal plasticity in the hippocampus.
These results suggest that nicotine-induced upregulation of gap-junctional proteins and ePSA might be independently involved in the repeated nicotine induced sensitization of the hippocampal complex.
References
1. Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001; 31:349–352. PMID: 11516393.

2. Shim I, Javaid JI, Wirtshafter D, Jang SY, Shin KH, Lee HJ, Chung YC, Chun BG. Nicotine-induced behavioral sensitization is associated with extracellular dopamine release and expression of c-Fos in the striatum and nucleus accumbens of the rat. Behav Brain Res. 2001; 121:137–147. PMID: 11275291.

3. Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996; 383:713–716. PMID: 8878480.

4. Freund RK, Luntz-Leybman V, Collins AC. Nicotine interferes with GABA-mediated inhibitory processes in mouse hippocampus. Brain Res. 1990; 527:286–291. PMID: 2253036.

5. Traub RD, Pais I, Bibbig A, LeBeau FE, Buhl EH, Hormuzdi SG, Monyer H, Whittington MA. Contrasting roles of axonal (pyramidal cell) and dendritic (interneuron) electrical coupling in the generation of neuronal network oscillations. Proc Natl Acad Sci U S A. 2003; 100:1370–1374. PMID: 12525690.

6. McCracken CB, Roberts DCS. Neuronal gap junctions: expression, function, and implications for behavior. Int Rev Neurobiol. 2006; 73:125–151. PMID: 16737903.

7. Colomer C, Olivos Ore LA, Coutry N, Mathieu MN, Arthaud S, Fontanaud P, Iankova I, Macari F, Thouënnon E, Yon L, Anouar Y, Guérineau NC. Functional remodeling of gap junction-mediated electrical communication between adrenal chromaffin cells in stressed rats. J Neurosci. 2008; 28:6616–6626. PMID: 18579734.

8. Tsai CH, Yeh HI, Tian TY, Lee YN, Lu CS, Ko YS. Down-regulating effect of nicotine on connexin43 gap junctions in human umbilical vein endothelial cells is attenuated by statins. Eur J Cell Biol. 2004; 82:589–595. PMID: 15035434.

9. Zhang TA, Tang J, Pidoplichko VI, Dani JA. Addictive nicotine alters local circuit inhibition during the induction of in vivo hippocampal synaptic potentiation. J Neurosci. 2010; 30:6443–6645. PMID: 20445070.
10. Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci. 2001; 21:1983–2000. PMID: 11245683.

11. Urban L, Neill KH, Crain BJ, Nadler JV, Somjen GG. Postischemic synaptic physiology in area CA1 of the gerbil hippocampus studied in vitro. J Neurosci. 1989; 9:3966–3975. PMID: 2585062.
12. Gill R, Kemp JA. Protection of CA1 pyramidal cell function by MK-801 following ischaemia in the gerbil. Neurosci Lett. 1989; 105:101–106. PMID: 2484724.

13. Yun SH, Park KA, Kwon S, Woolley CS, Sullivan PM, Pasternak JF, Trommer BL. Estradiol enhances long term potentiation in hippocampal slices from aged apoE4-TR mice. Hippocampus. 2007; 17:1153–1157. PMID: 17696167.

14. Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006; 26:8517–8522. PMID: 16914677.

15. Perez J, Tranque PA, Naftolin F, Garcia-Segura LM. Gap junctions in the hypothalamic arcuate neurons of ovariectomized and estradiol-treated rats. Neurosci Lett. 1990; 108:17–21. PMID: 2304626.

16. Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004; 24:495–499. PMID: 14724248.

17. Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003; 23:1588–1592. PMID: 12629162.

18. Matsumoto A, Arnold AP, Zamphigi GA, Micevych PE. Androgenic regulation of gap junction between motoneurons in the rat spinal cord. J Neurosci. 1988; 8:4177–4183. PMID: 3183718.
19. Matsuyama S, Matsumoto A, Enomoto T, Nishizaki T. Activation of nicotinic acetylcholine receptors induces long-term potentiation in vivo in the intact mouse dentate gyrus. Eur J Neurosci. 2000; 12:3741–3747. PMID: 11029644.
20. Harley CE, Malsbury CW, Squires A, Brown RAM. Testosterone decreases CA1 plasticity in vivo in gonadectomized male rats. Hippocampus. 2000; 10:693–697. PMID: 11153715.

21. Hervé JC, Bourmeyster N, Sarrouilhe D, Duffy HS. Gap junctional complexes: from partners to functions. Prog Biophys Mol Biol. 2007; 94:29–65. PMID: 17507078.

22. Oyamada M, Oyamada Y, Takamatsu T. Regulation of connexin expression. Biochim Biophys Acta. 2005; 1719:6–23. PMID: 16359940.

Fig. 1
Illustrations of the recording of evoked population spike amplitude (ePSA). (A) Position of stimulating and recording electrodes in the hippocampus CA1 area. (B) Patterns of evoked population spikes recorded in different levels of the pyramidal neuron. (C) Dose-dependent nicotine effect on ePSA in male gerbils. Saline, saline, 1 ml/kg i.p. injection; NT-0.5, nicotine bitartrate, 0.5 mg/kg, i.p. injection; NT-5.0, nicotine bitartrate, 5.0 mg/kg, i.p. injection.
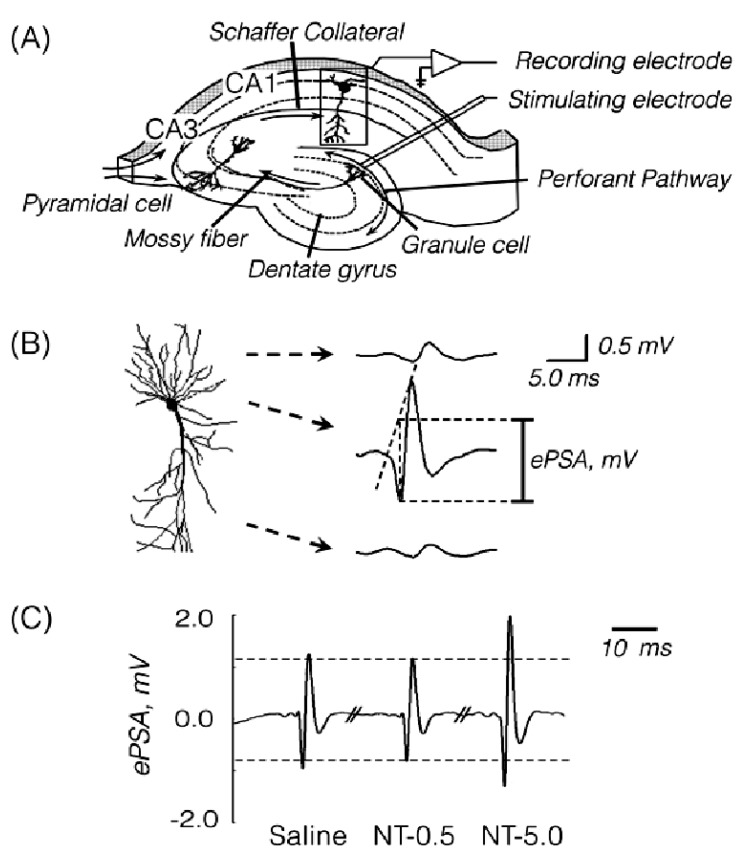
Fig. 2
Acute and repeated nicotine effects on evoked population spike amplitude. (A) Sex-related difference in the response of ePSA to NT-0.5 and NT-5.0, respectively. Symbols: ○, saline, 1 ml/kg i.p. injection; •, NT-0.5: nicotine bitartrate, 0.5 mg/kg, i.p. injection; ▴, NT-5.0: nicotine bitartrate, 5.0 mg/kg, i.p. injection. (B) Responses of ePSA to a challenging NT-0.5 in male and female gerbils pretreated with repeated injections of NT-0.5 s.c., twice per day for 7 days or 28 days. ○, acute NT-0.5 injection; •, pretreatment with NT-0.5 for 7 days; ▴, pretreatment with NT-0.5 for 28 days. Each bar represents the mean±SE (n=5 or 6). * and ** indicate p<0.05 and p<0.01, respectively, compared to control groups: (A) saline group and (B) NT-0.5 group.
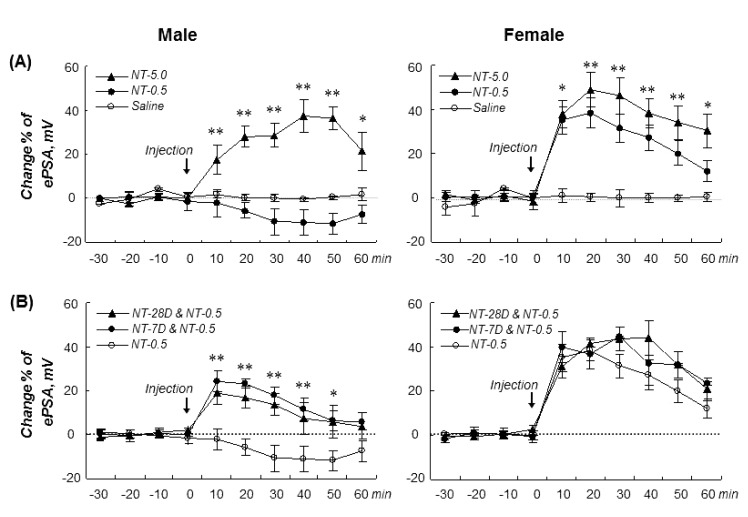
Fig. 3
Double immunohistochemistry photographs. (A) The oriens (Or), pyramidal (Py) and radiatum (Rad) layers in the hippocampus CA1 area (×200); (B) anti-NeuN immunoreactive neurons, (C) anti-Cx36 immunoreactive dots; (D) merged image of B and C (×600). Scale bars: (A) 50 µm and (D) 7 µm.
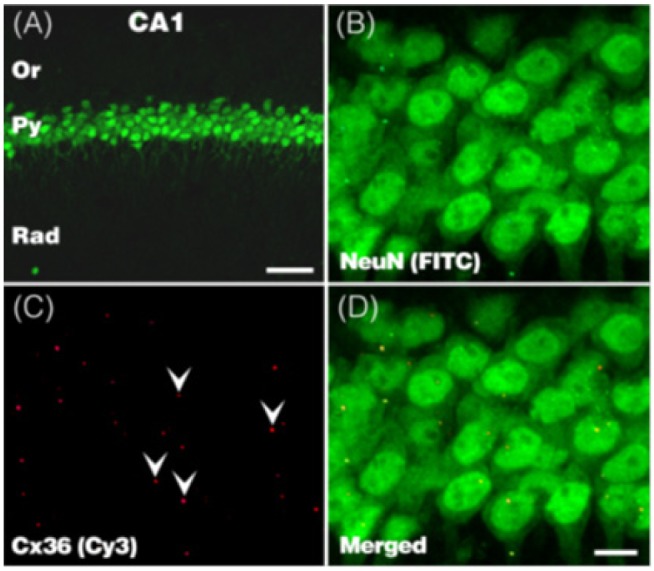
Fig. 4
Increases of (A) anti-Cx36 and (B) anti-Cx43 IR dots by a challenging NT-0.5 in gerbils pretreated with repeated NT-0.5 injections twice a day for 7 days. The following data were obtained from 5 animals by blind counting in a predefined region (300×400 µm2) of the pyramidal layer in serial 16 images per animal. * and ** indicate p<0.05 and p<0.01, respectively, in comparison to the saline treated group. †Means p<0.05, with respect to comparisons between male and female groups. anti-Cx36, -Cx43 dots, total count of anti-Cx36, -Cx43 IR dots; anti-Cx36, -Cx43 dots/Neuron, count of anti-Cx36, -Cx43 IR dots per an anti-NeuN IR neuron; anti-Cx36, -Cx43 IR+Neurons, count of anti-NeuN IR neurons with anti-Cx36, -Cx43 IR dots. SS & SN, Saline & NT-0.5 challenge after repeated saline pretreatment for 7 days; NS & NN, Saline & NT-0.5 challenge after repeated NT-0.5 pretreatment for 7 days.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download