INTRODUCTION
METHODS
Animal preparation
Extracellular recording and electrical stimulation
Artifact removal and data analysis
 | Fig. 1Protocol for artifact removal with a curve-fitting method. (A) Single unit activities are covered by large stimulus artifact waveforms. (B) An example of one artifact waveform at a stimulation frequency of 10 Hz. Several spikes were mixed on the fluctuation generated by an artifact waveform (indicated by arrows). (C) The artifact waveform is divided into several segments and each segment is curve-fitted with 2nd to 4th order polynomial. Every curve-fitted waveform is gathered together to construct a template for artifact subtraction. (D) Spike waveforms were clearly revealed after artifact subtraction (the template is subtracted from the original waveforms). (E) Every artifact waveform is subtracted by templates, which are curve-fitted from their own waveform. After this procedure is repeated, an artifact-removed signal is obtained. |
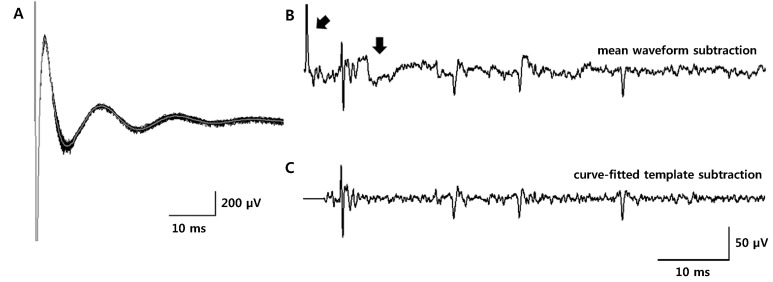 | Fig. 2Comparison of two artifact removal methods. (A) 100 overlapped artifact waveforms (thick gray trace: mean waveform) recorded with a stimulation frequency of 10 Hz. (B) Artifact-removed waveform after the mean waveform subtraction. Residual artifacts and fluctuation remained (indicated by arrows). (C) Artifact-removed waveform after the template subtraction with the curve-fitting method. Residual artifacts that are shown in Fig. 2 (B) were not generated using the curve-fitting method. |
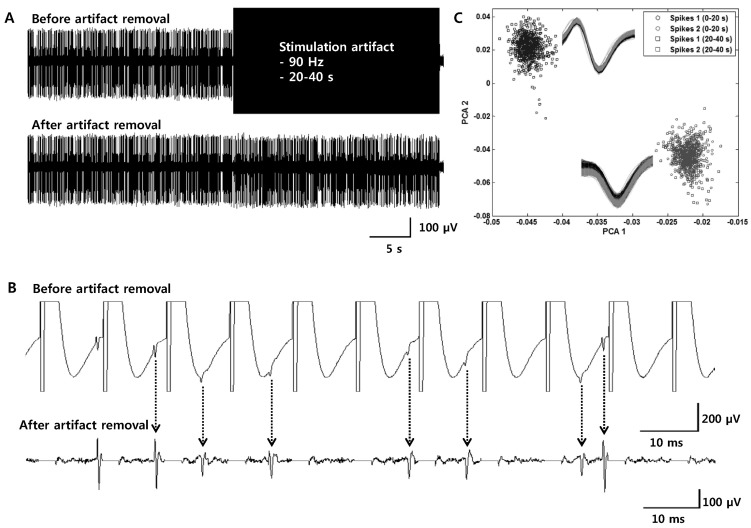 | Fig. 3Simulations for the validation of the effectiveness of the artifact removal technique with the curve-fitting method. (A, B) Two different shapes of spikes were randomly synthesized to the noise (first 20 seconds) and to the noise and artifact waveforms (second 20 seconds). In this figure, stimulation frequency of the artifacts was 90 Hz (top: before artifact removal, bottom: after artifact removal). (C) Spikes are detected after artifact removal and classified by principal component analysis (PCA). Spikes detected before and after artifact subtractions have similar spike waveforms and can be reliably classified into two groups (In the box: spikes waveforms sorted by PCA analysis. Black waveforms: spikes detected from the first 20 seconds. Grey waveforms: spikes detected from the second 20 seconds after artifacts were removed). |
RESULTS
Simulation of artifact removal
Responses of GP neurons during STN stimulation
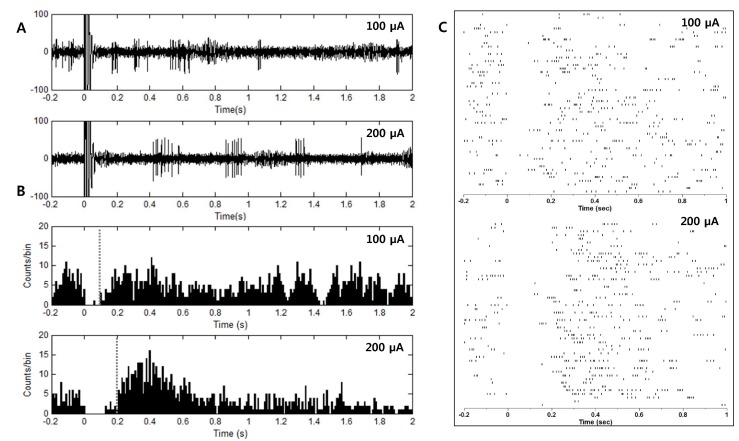 | Fig. 4Inhibition of neuronal activity of GP neurons by STN stimulation. (A) Inhibition of neuronal activity followed electrical stimulation. Stimulation of a larger amplitude induced longer inhibition (top: 100 µA, bottom: 200 µA). (B, C) A post-stimulus time histogram (PSTH, time bin: 10 ms) and raster plot were constructed from 50 repetitive stimulations. The duration of the inhibition was approximately 100 ms and 200 ms for pulse amplitude 100 µA and 200 µA, respectively. |
 | Fig. 5Waveforms recorded from GP neurons of Parkinson's disease model rats. (A) Electrical stimulations were applied to the STN with various stimulation frequencies (from the top: 10, 50, 90 and 130 Hz). (B) Artifact-removed waveforms. Artifact-removed periods are indicated with boxes of dotted lines. (C) Spike waveforms detected from each waveform. Two units were detected and sorted. Spikes with amplitude larger than another unit showed inhibition of firing rate when high frequency stimulation was applied to the STN. |
 | Fig. 6Changes in the firing rate according to the stimulation frequency. Changes of the mean firing rate during electrical stimulation were compared to the mean firing rate 10 s before the stimulation to obtain quantitative values (relative change, %). Both in (A) normal and (B) PD model rat, significant inhibition of neuronal firing was observed at stimulation frequencies higher than 50 Hz. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download