1. Wattanapitayakul SK, Bauer JA. Oxidative pathways in cardiovascular disease: roles, mechanisms, and therapeutic implications. Pharmacol Ther. 2001; 89:187–206. PMID:
11316520.

2. Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008; 88:581–609. PMID:
18391174.

3. Hearse DJ, Bolli R. Reperfusion induced injury: manifestations, mechanisms, and clinical relevance. Cardiovasc Res. 1992; 26:101–108. PMID:
1571929.

4. Gross GJ, Kersten JR, Warltier DC. Mechanisms of postischemic contractile dysfunction. Ann Thorac Surg. 1999; 68:1898–1904. PMID:
10585101.

5. Verma S, Fedak PW, Weisel RD, Butany J, Rao V, Maitland A, Li RK, Dhillon B, Yau TM. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002; 105:2332–2336. PMID:
12021216.

6. Hardoon SL, Whincup PH, Lennon LT, Wannamethee SG, Capewell S, Morris RW. How much of the recent decline in the incidence of myocardial infarction in British men can be explained by changes in cardiovascular risk factors? Evidence from a prospective population-based study. Circulation. 2008; 117:598–604. PMID:
18212284.
7. Luepker RV. Decline in incident coronary heart disease: why are the rates falling? Circulation. 2008; 117:592–593. PMID:
18250277.
8. Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005; 14:170–175. PMID:
16009313.

9. Kim JH. Cardiovascular diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J Ginseng Res. 2012; 36:16–26. PMID:
23717100.

10. Dhar ML, Dhar MM, Dhawan BN, Mehrotra BN, Ray C. Screening of indian plants for biological activity: I. Indian J Exp Biol. 1968; 6:232–247. PMID:
5720682.
11. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the zutphen elderly study. Lancet. 1993; 342:1007–1011. PMID:
8105262.

12. Lee H, Kim J, Lee SY, Park JH, Hwang GS. Processed
Panax ginseng, Sun Ginseng, decreases oxidative damage induced by
tert-butyl hydroperoxide via regulation of antioxidant enzyme and Anti-apoptotic Molecules in HepG2 Cells. J Ginseng Res. 2012; 36:248–255. PMID:
23717125.
13. Yamabe N, Song KI, Lee W, Han IH, Lee JH, Ham J, Kim SN, Park JH, Kang KS. Chemical and free radical-scavenging activity changes of ginsenoside re by maillard reaction and its possible use as a renoprotective agent. J Ginseng Res. 2012; 36:256–262. PMID:
23717126.

14. Park JD. Recent studies on the chemical constituents of Korean ginseng (Panax ginseng C.A. Meyer). Korean J Ginseng Sci. 1996; 20:389–415.
15. Kim JH. Protective roles of ginseng saponin in cardiac ischemia and reperfusion injury. J Ginseng Res. 2009; 33:283–293.

16. Massoudy P, Becker BF, Gerlach E. Bradykinin accounts for improved postischemic function and decreased glutathione release of guinea pig heart treated with the angiotensin-converting enzyme inhibitor ramiprilat. J Cardiovasc Pharmacol. 1994; 23:632–639. PMID:
7516015.

17. Massoudy P, Beblo S, Raschke P, Zahler S, Becker BF. Influence of intact left atrial appendage on hemodynamic parameters of isolated guinea pig heart. Eur J Med Res. 1998; 3:470–474. PMID:
9753704.
18. Guo L, Dong Z, Guthrie H. Validation of a guinea pig Langendorff heart model for assessing potential cardiovascular liability of drug candidates. J Pharmacol Toxicol Methods. 2009; 60:130–151. PMID:
19616638.

19. Minematsu T, Ohtani H, Yamada Y, Sawada Y, Sato H, Iga T. Quantitative relationship between myocardial concentration of tacrolimus and QT prolongation in guinea pigs: pharmacokinetic/pharmacodynamic model incorporating a site of adverse effect. J Pharmacokinet Pharmacodyn. 2001; 28:533–554. PMID:
11999291.
20. Ohtani H, Hanada E, Yamamoto K, Sawada Y, Iga T. Pharmacokinetic-pharmacodynamic analysis of the electrocardiographic effects of terfenadine and quinidine in rats. Biol Pharm Bull. 1996; 19:1189–1196. PMID:
8889039.

21. Asha S, Radha E. Effect of age and myocardial infarction on serum and heart lactic dehydrogenase. Exp Gerontol. 1985; 20:67–70. PMID:
3996487.

22. Gerhardt W, Ljungdahl L, Herbert AK. Troponin-T and CK MB (mass) in early diagnosis of ischemic myocardial injury. The Helsingborg Study, 1992. Clin Biochem. 1993; 26:231–240. PMID:
8242886.

23. Bertinchant JP, Larue C, Pernel I, Ledermann B, Fabbro-Peray P, Beck L, Calzolari C, Trinquier S, Nigond J, Pau B. Release kinetics of serum cardiac troponin I in ischemic myocardial injury. Clin Biochem. 1996; 29:587–594. PMID:
8939408.

24. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358. PMID:
36810.

25. Beutler E. The glutathione instability of drug-sensitive red cells; a new method for the in vitro detection of drug sensitivity. J Lab Clin Med. 1957; 49:84–95. PMID:
13385579.
26. Molavi B, Mehta JL. Oxidative stress in cardiovascular disease: molecular basis of its deleterious effects, its detection, and therapeutic considerations. Curr Opin Cardiol. 2004; 19:488–493. PMID:
15316458.

27. Di Meo S, Venditti P, De Leo T. Tissue protection against oxidative stress. Experientia. 1996; 52:786–794. PMID:
8774749.

28. Wattanapitayakul SK, Bauer JA. Oxidative pathways in cardiovascular disease: roles, mechanisms, and therapeutic implications. Pharmacol Ther. 2001; 89:187–206. PMID:
11316520.

29. Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006; 70:181–190. PMID:
16580655.

30. Peng Y, Sun Z. Characterization of QT and RR interval series during acute myocardial ischemia by means of recurrence quantification analysis. Med Biol Eng Comput. 2011; 49:25–31. PMID:
20725861.

31. Kaul N, Siveski-Iliskovic N, Hill M, Slezak J, Singal PK. Free radicals and the heart. J Pharmacol Toxicol Methods. 1993; 30:55–67. PMID:
8298182.

32. Robicsek F, Schaper J. Reperfusion injury: fact or myth? J Card Surg. 1997; 12:133–137. PMID:
9395941.

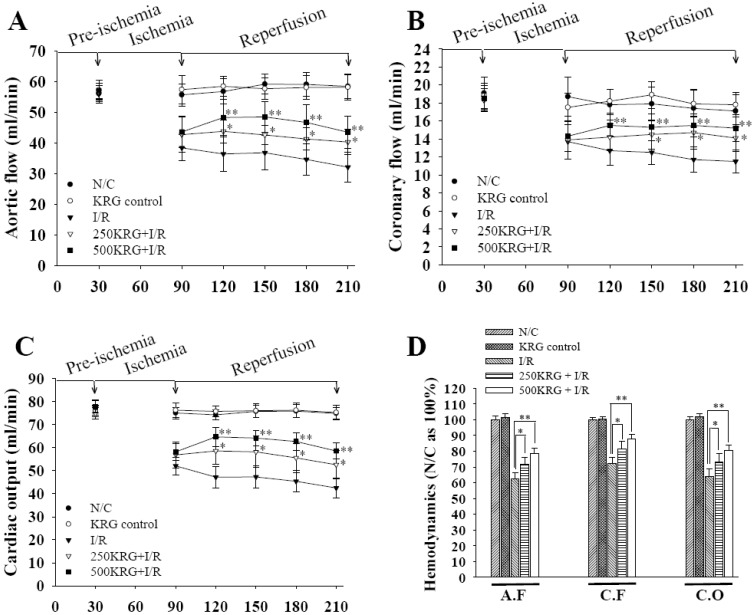
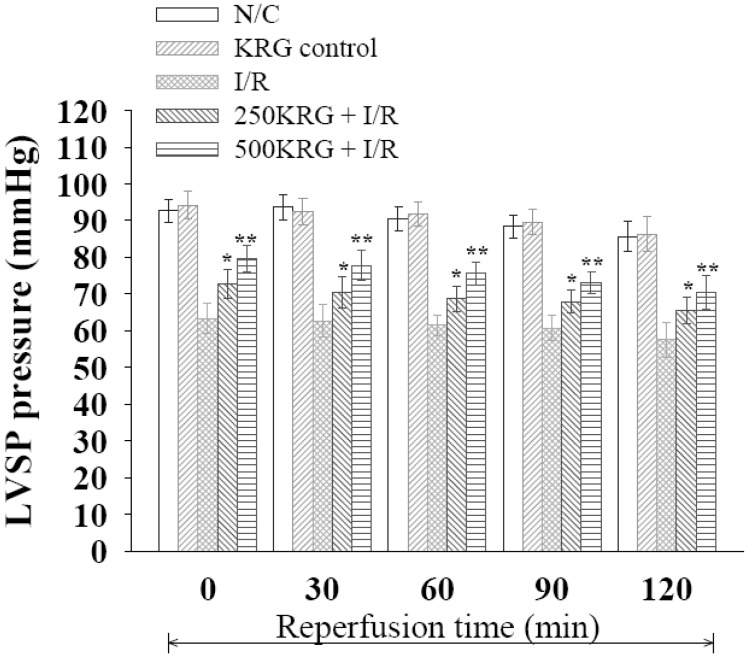
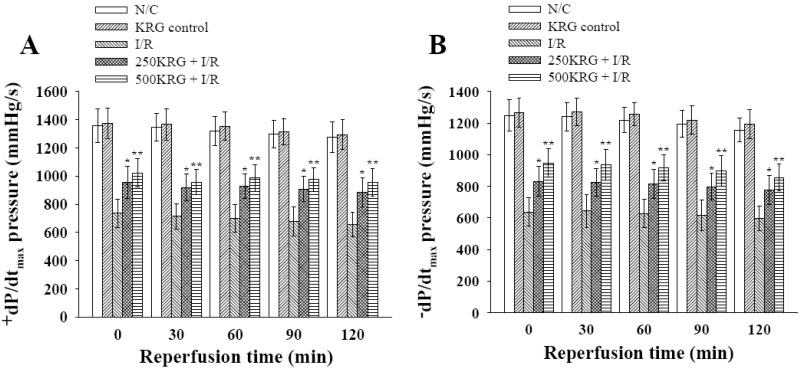
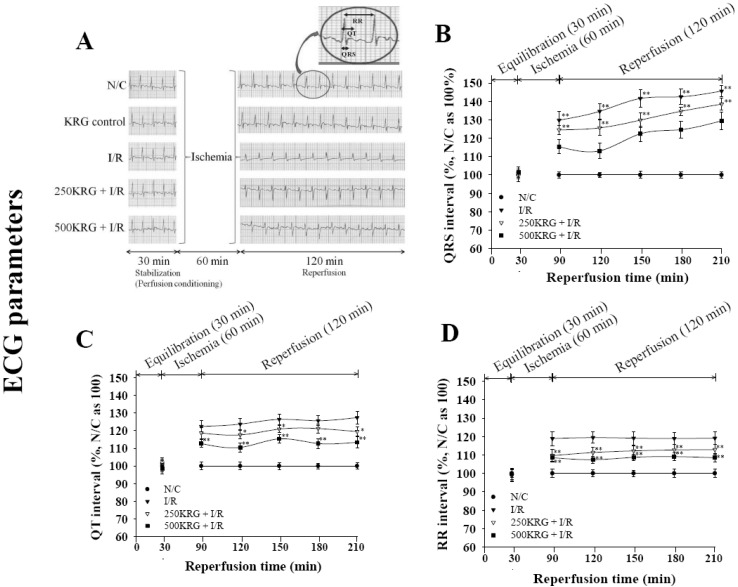




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download