INTRODUCTION
METHODS
Animals
In-vivo ERG recording
Analysis of ERG recordings
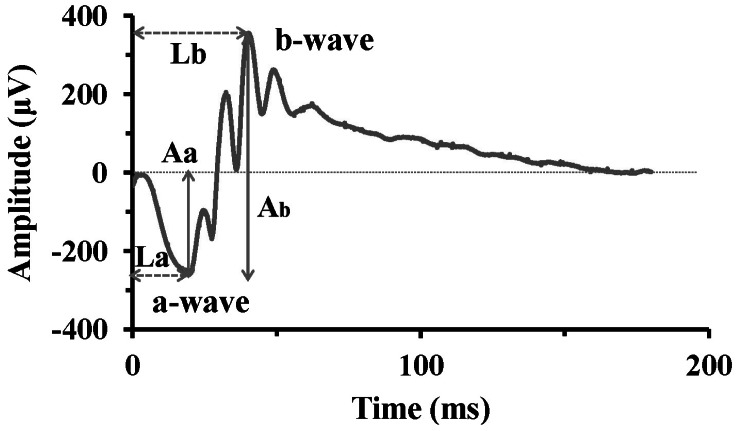 | Fig. 1Typical ERG waveform. The initial negative component, a-wave represents photoreceptor response, while the b-wave, positive peak following the a-wave, represents activities of bipolar cells or Müller cells. The a-wave amplitude (Aa) is measured from the baseline to the a-wave trough, and the b-wave amplitude (Ab) from the a-wave trough to the b-wave peak. The implicit time of a-wave (La) and b-wave (Lb) is measured from the stimulus onset to the a-wave trough and b-wave peak, respectively. |
Ex-vivo retinal preparation
Electrode and data recording system for ex-vivo recording
MEA data analysis
Retinal tissue preparation for staining
Cresyl violet and TUNEL staining
RESULTS
Comparison of ERG waveforms in wt mice and rd10 mice
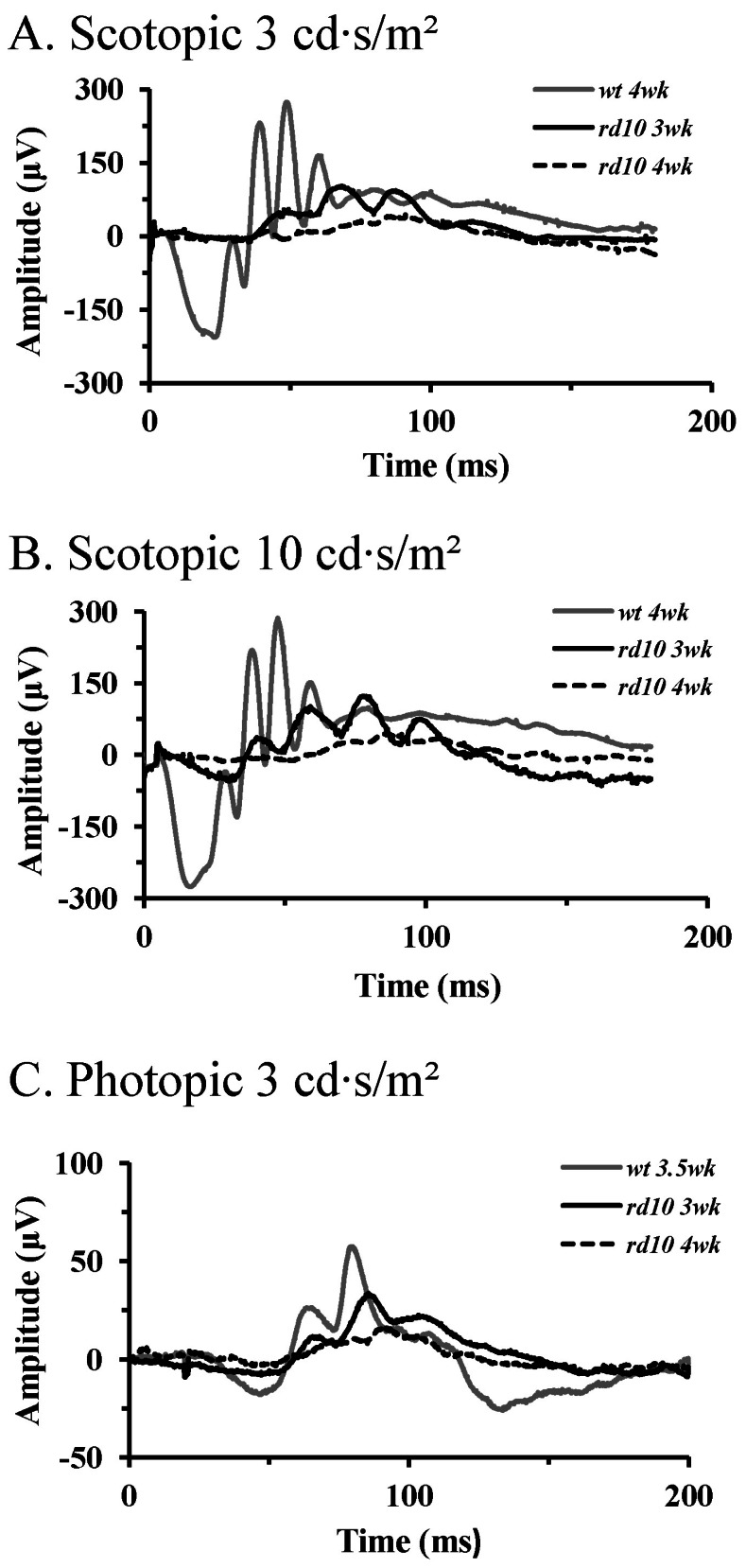 | Fig. 2ERG recordings in wt mice and rd10 mice. (A) Light stimulus condition of scotopic 3 cd·s/m2. (B) Light stimulus condition of scotopic 10 cd·s/m2. (C) Light stimulus condition of photopic 3 cd·s/m2. Dark-adapted responses were recorded after 1 hour of darkad-aptation. Note the increase in amplitudes of the a- and b-wave with increasing light intensities. At the same light intensities, response of the rd10 mice are delayed and reduced in amplitude compared with those of the wt mice. Unlike in wt mice, a-wave in rd10 mice was hardly detectable in postnatal 4 weeks. |
Comparison of ERG b-wave in wt mice and rd10 mice
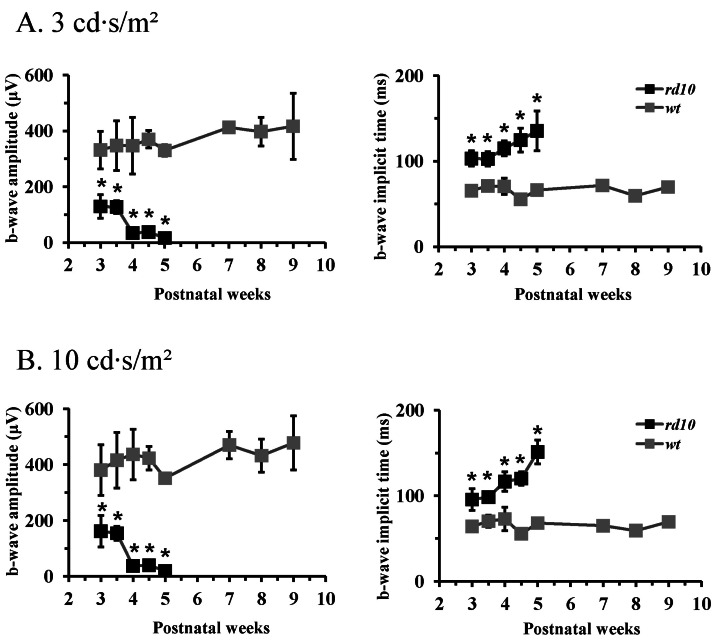 | Fig. 3ERG b-wave of age-matched wt mice and rd10 mice. (A) Light stimulus condition of scotopic 3 cd·s/m2. (B) Light stimulus condition of scotopic 10 cd·s/m2. The b-wave amplitude of rd10 mice is significantly smaller and the implicit time of b-wave is significantly longer than that of wt mice throughout all the postnatal ages. Each point represents the mean±standard deviation (S.D.) (*p<0.001). |
Spontaneous retinal ganglion cell (RGC) spikes and local field potential at different postnatal ages
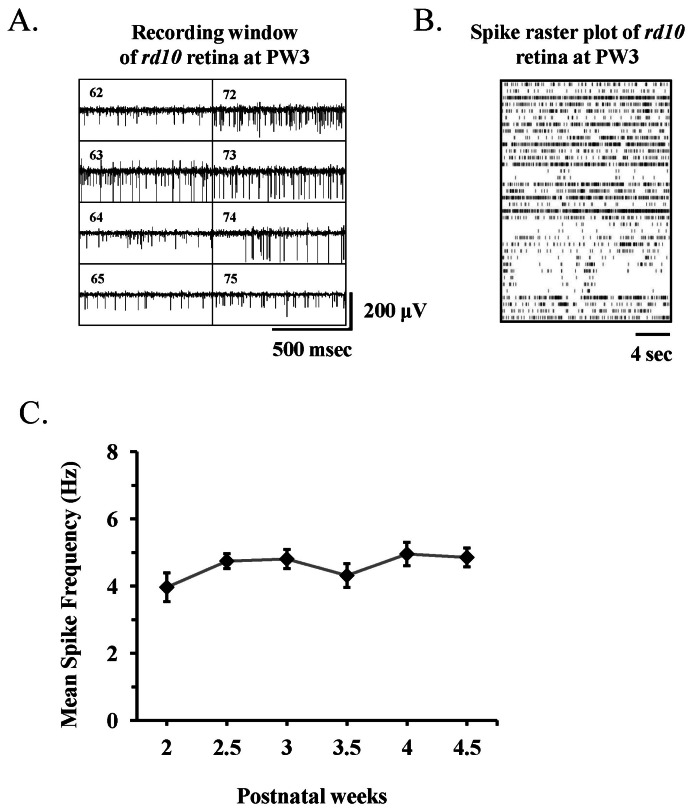 | Fig. 4Spontaneous retinal activity in rd10 mouse recorded with multichannel recording system. (A) Part of real time recording window of data acquisition software (MC_Rack) shows 8 channels of 8×8 multielectrode array. The channel number is shown inside of each channel (e.g., channel 62 means the channel of 6th column and 2nd row). Rd10 mouse at postnatal 3 week (PW3) were used. (B) Raster plots of 36 retinal ganglion cell (RGC) spikes (One RGC spike train per each row of plot) recorded from single rd10 retina at PW3. (C) Mean±standard error of the mean (SEM) firing rate of all cells recorded across different postnatal weeks. There is no statistically significant difference among different postnatal weeks (p>0.05). |
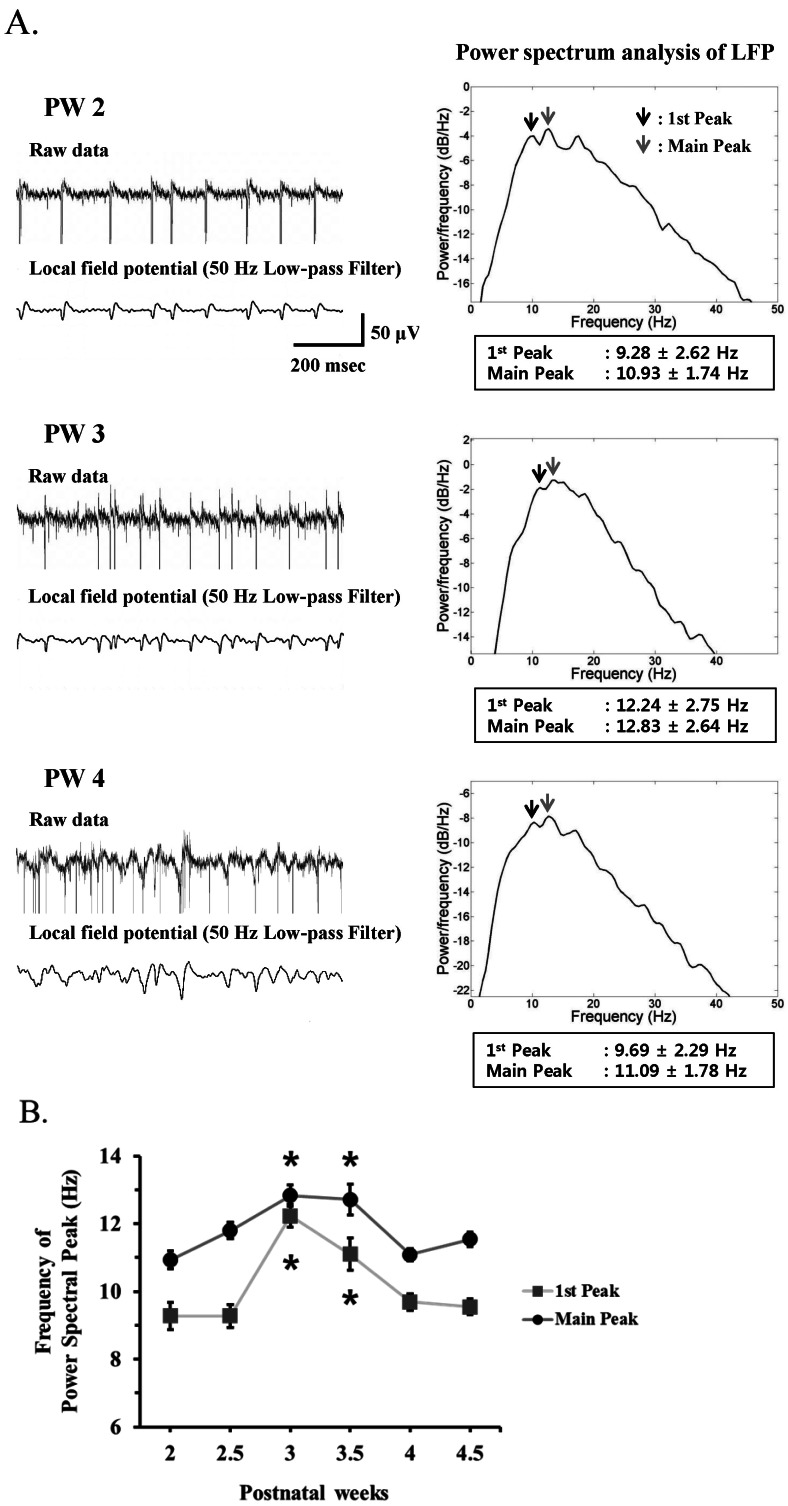 | Fig. 5(A) Left panel. Upper trace: A typical raw waveform of neural activity recorded from postnatal week 2 (PW2), PW3, and PW4 rd10 retina. Lower trace: Local field potential (LFP) waveform obtained from low-pass filtering with 50 Hz cutoff frequency. Right panel: Power spectrum analysis of LFP estimated by the Burg algorithm was shown. Arrows indicate the 1st and main spectral peak. (B) Frequency of power spectral peak of LFP across different postnatal weeks. The 1st and main peak of LFP at PW3 and PW3.5 are significantly different with other age groups (*p<0.05, ANOVA and posthoc Tukey criteria). At each postnatal week, 3 rd10 mice were used. The number of MEA channels for analysis was 44, 87, 68, 50, 95, and 86 at PW2, PW2.5, PW3, PW3.5, PW4, and PW4.5, respectively. Error bars are ±SEM. |
Morphological changes in different postnatal-aged retina of rd10 mice
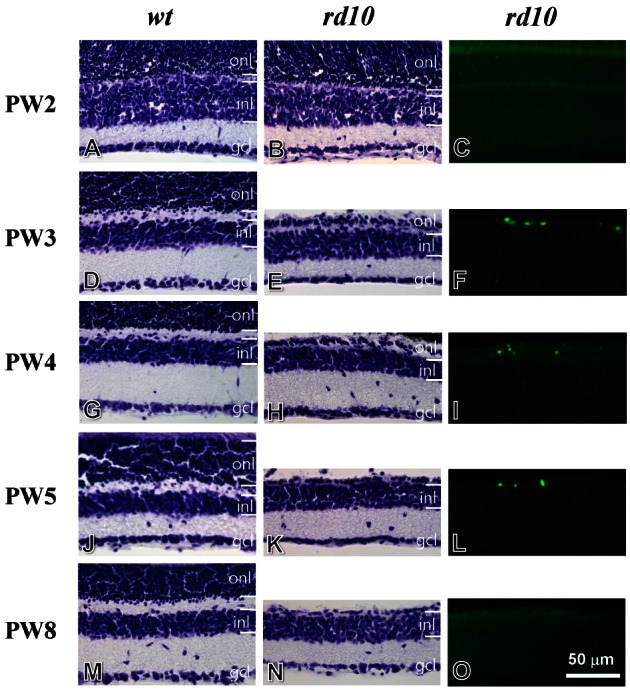 | Fig. 6Cresyl violet staining and TUNEL staining for developing retinae of wt mice (left panel), rd10 mice (middle and right panels). (A~C) Postnatal week 2 (PW2). The retina of the rd10 mice has intact layers, identical to that of the wt mice (A, B). Few TUNEL-stained cells are observed in the rd10 retina (C). (D~F) PW3. Compared to the wt retina (D), the thickness of outer nuclear layer (ONL) is significantly reduced in the rd10 retina (E). A number of TUNEL-stained cells are seen in the ONL of the rd10 retina (F). (G~I) PW4. The morphology of the rd10 retina is similar to that of the PW3 rd10 retina (G~I). (J~L) PW5. Compared to the wt retina (J), one or two row(s) of cells remain(s) in the ONL of the rd10 retina (K). A few TUNEL-stained cells are seen in the ONL of the rd10 retina (L). (M~O) PW8. The morphology of the rd10 retina is similar to the PW5 rd10 retina (M, N). However, TUNEL-stained cells were not detected in the rd10 retina (O). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar=50 µm. |
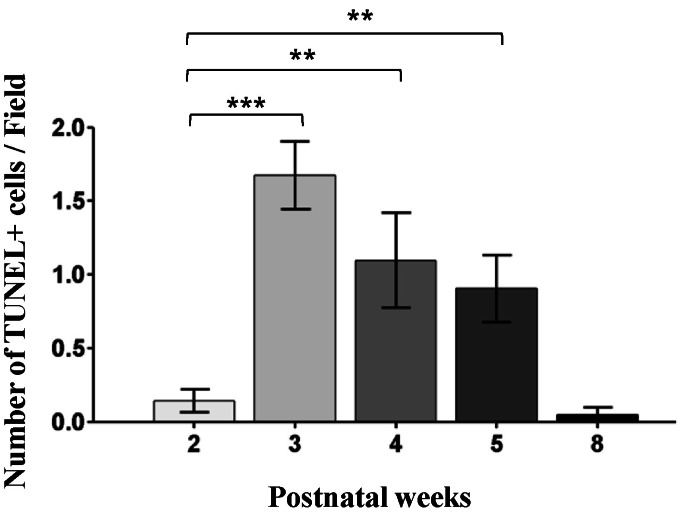 | Fig. 7The number of the TUNEL-stained cells in the developing retinae of the rd10 mice. In comparison to the PW2 rd10 retina, the number of TUNEL-stained cells is significantly increased in the PW3 to PW5 rd10 retinae (***p<0.001, **p<0.01). However, the number of TUNEL-stained cells is reduced in the PW8 rd10 retina. Note that TUNEL-stained cells are most frequently observed in the PW3 rd10 retina. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download