INTRODUCTION
Calcium-activated K
+ (BK
Ca) channels are high-conductance, voltage-dependent, and potassium-selective ion channels. BK
Ca channels are activated by depolarization and elevation of intracellular Ca
2+ levels and causes a closure of voltage-dependent Ca
2+ channels and reduces Ca
2+ influx into the cell. Thus, BK
Ca channels are dually regulated through membrane voltage and intracellular Ca
2+. BK
Ca channels comprise two subunits, α and β [
1-
3]. Ca
2+-dependent kinases also can phosphorylate the α subunit and regulate the channel activity [
4]. BK
Ca channels participate in diverse physiological processes such as neuronal excitability, smooth muscle contractility, hair cell tuning, and have a protective mechanism against ischemic cell death of neurons [
5]. In addition, there are two regulators of K
+ conductance, the RCK1 and RCK2 domains in the cytosolic domain of the BK
Ca channel [
6]. These contain two high affinity Ca
2+ binding sites. One is in the RCK1 domain at position Asp362/367 and the other is in the Ca
2+ bowl that contains a series of Asps located in the RCK2 domain [
7,
8] (
Fig. 1A).
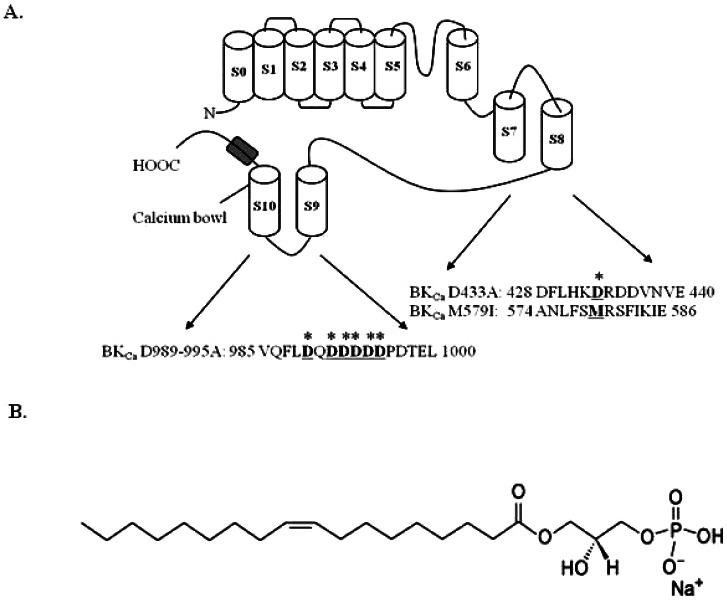 | Fig. 1Schematic transmembrane diagram of the BK Ca channel in the plasma membrane and the LPA chemical structure. (A) There are 7 transmembrane domains along with the RCK1 and RCK2 domains. The voltage sensor and pore of the BK Ca channel resembles the responding regions of other voltage-dependent K + channels ( Yuan et al., 2010). Amino acid residues underlined and marked (*) indicate mutation sites in this study. (B) The chemical structure of lysophosphatidic acid (LPA, C 18:1). 
|
Lysophosphatidic acid (LPA, 1-radyl-2-hydroxy-
sn-glycero-3-phosphate) is the prototypical member of the family of lipid mediators [
9] (
Fig. 1B). The endothelial gene (EDG) family encodes for the GPCR-specific LPA and S1P [
9]. LPA activates diverse cellular actions through interactions with the G protein-coupled LPA receptors, which are responsible for the transient elevation of intracellular Ca
2+ levels [
9-
11]. In addition, it was demonstrated that neurite retraction and cell rounding in cell lines of neural origin are caused by LPA [
12]. Applying LPA extracellularly induces cell polarization, directional motility, and metabolic burst in human neutrophils [
13]. However, relatively little is known about how LPA regulates ion channel activity, especially in Ca
2+-dependent ion channels.
In the present study, we examined the effects of LPA on BKCa (rat brain rSlo) channel activities expressed in Xenopus laevis oocytes by using the two-electrode voltage clamp technique. We found that LPA activates the BKCa channel in a concentration- and voltage-dependent manner. Moreover, when we treated the cells with LPA repeatedly, we could observe the desensitization of LPA-mediated BKCa channel activation. By using various inhibitors of phospholipase C, IP3 receptor, and protein kinase C, we examined the signaling pathway in LPA-mediated BKCa channel activation. In addition, the effects of site-directed mutagenesis on RCK1 and mutations in the Ca2+ bowl in LPAmediated BKCa channel activation were examined. Finally, we have discussed the physiological or pharmacological roles of LPA in the nervous and vascular systems.
Go to :

RESULTS
Treatment of LPA in oocytes injected with cRNA encoding the BK
Ca α subunit elicited a large outward current with a voltage step from -80 to +40 mV with a 400 ms duration at 10-s intervals (
Fig. 2A). We next examined the effects of various concentrations of LPA on the BK
Ca channel activity. As shown in
Fig. 2, the application of LPA on oocytes induced BK
Ca channel activation in a concentration-dependent manner. Charybdotoxin and iberiotoxin, highly specific inhibitor of maxi-K channels [
18,
19], greatly attenuated the BK
Ca channel activations induced by LPA (data not shown), indicating that BK
Ca channels are functionally working [
20]. Oocytes injected with only H
2O elicited a basal outward current (data not shown). LPA activated the BK
Ca channel by an average of 761.42±124.10% at 1 µM. The EC
50 was 0.12±0.05 µM (
Fig. 2Ab). These results indicate that LPA induces BK
Ca channel activation.
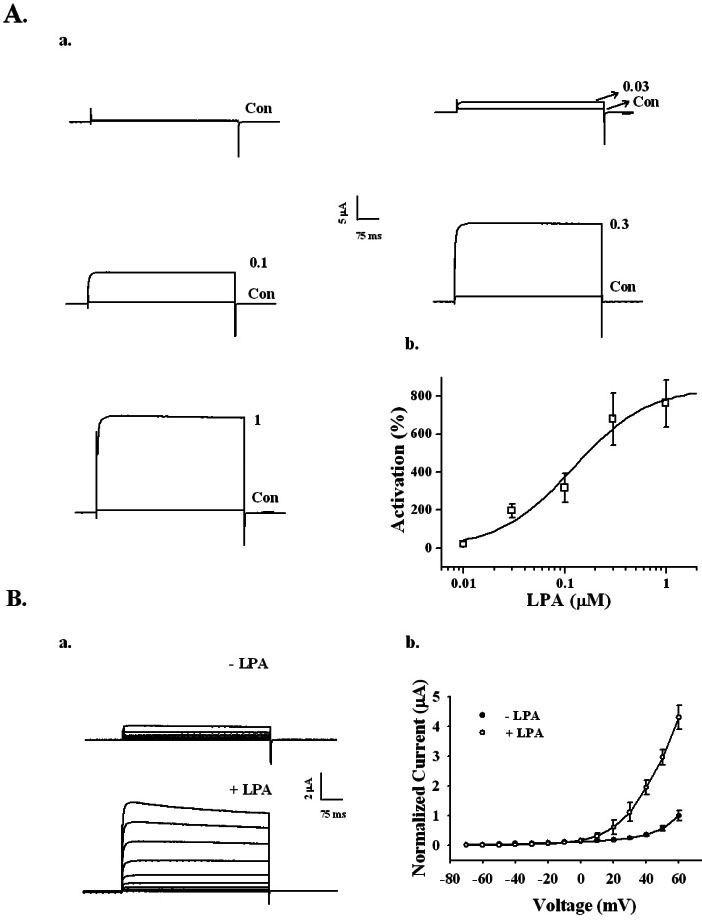 | Fig. 2Concentration- and voltage-dependent activation of the BKCa channel by LPA in Xenopus oocytes expressing rat brain BKCa channels. (A) The BKCa channel activation by LPA is concentration-dependent. (a) Representative traces of BKCa channel current enhancements using different concentrations of LPA (up to 1 µM). (b) The summary histogram shows the effects of various concentrations of LPA on the BKCa channels. (B) A representative control and LPA-mediated current in an I~V relationship. (a) Currents were recorded at a test potential from -70 to +60 mV, applied from a holding potential of -80 mV. (b) Current-voltage (I-V) relationship of the BKCa channel in the absence (●) or presence (○) of 100 nM LPA. Voltage pulses with 400-ms duration were applied in 10 mV increments at 3-s intervals from a holding potential of -80 mV. The current peaks, normalized to the peak current evoked by the voltage step to +60 mV in the absence of LPA, were used in the I-V plot. Data represent the mean±S.E.M. (n=4). 
|
The effect of LPA on the current-voltage (I~V) relationship was examined. The current response evoked by each voltage step (i.e., a series of voltage pulses of 400 ms duration given in 10 mV increments and 3-s intervals from the holding potential of -80 mV) was used to obtain the I~V curve. In the absence of LPA, voltage pulses greater than 0 mV exhibited slight BK
Ca channel currents (
Fig. 2Ba,
upper traces). The presence of LPA increased the current amplitude over 0 mV compared to the absence of LPA (
Fig. 2Ba,
lower traces), indicating that LPA activates the BK
Ca channel in a voltage-dependent manner (
Fig. 2Bb).
We examined the possible changes caused by LPA on BK
Ca channel currents after repeated applications of LPA. As shown in
Fig. 3A, treatment with 100 nM LPA for 1 min enhanced the BK
Ca channel current. Oocytes were then washed with recording buffer for 3 min. When the outward current returned to the basal level, oocytes were re-treated with LPA. The second, third, and fourth BK
Ca current responses for LPA were significantly diminished. The BK
Ca currents diminished as follows: 410±25, 137±25, 34.6±10.3, 2±1.25%, for the first, second, third, and forth response, respectively (n=5, oocytes from two different batches of donors), indicating that repeated treatment of LPA desensitized BK
Ca channel activation.
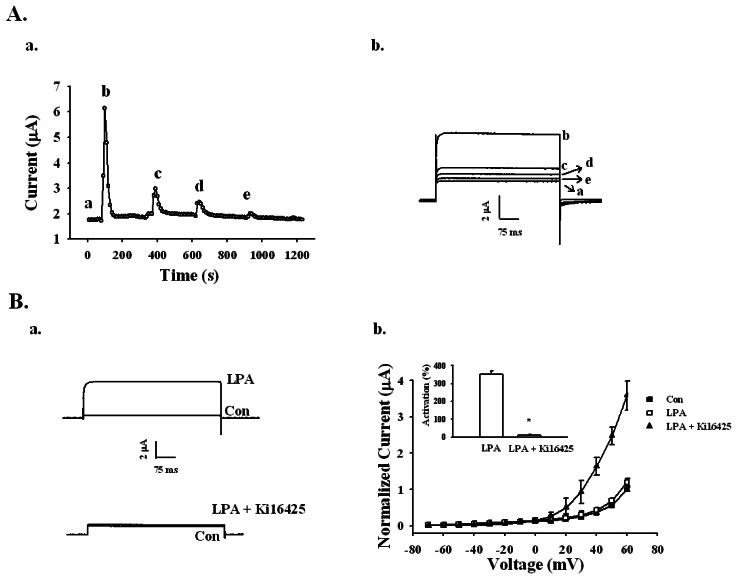 | Fig. 3Effects of repeated treatments of LPA and LPA1/3 receptor antagonist Ki16425 on LPA-mediated BKCa channel activation. (A-a) Repeated application of 100 nM LPA desensitized the BKCa channel activation with indicated time interval in representative oocyte (left panel). (A-b) The representative BKCa channel current traces by repeated LPA treatments were obtained from timecurrent relationship of "a" (right panel). Outward currents are caused by stepping from -80 mV to +40 mV for 400 ms. Data represent the mean±S.E.M. (n=5). (B-a) Representative traces are from the LPA channel activation in the absence (upper trace) or presence of 10 µM Ki16425 for 10 min (lower trace). (B-b) Summary histogram of the LPA effects on BKCa channel activation (right panel). Data represent the mean±S.E.M. (n=10~12; *p<0.01). 
|
Since the previous report showed that
Xenopus oocytes express the endogenous LPA1 receptor [
21], we examined whether the LPA receptor 1/3 antagonist Ki16425 could inhibit LPA-induced BK
Ca channel activation. Oocytes were first incubated with 10 µM Ki16425 for 10 min. In the absence of Ki16425, LPA (100 nM) activated the BK
Ca channel by 350.52±21.91%; however, the presence of Ki16425 LPA (100 nM) activated the BK
Ca channel by only 12.50±2.53% (
Fig. 3B). Thus, LPA activates the BK
Ca channel through LPA receptor activation.
We next investigated the signal transduction pathway for LPA-mediated activation of BK
Ca channels. First, we used an active PLC inhibitor, U-73122, and an inactive PLC inhibitor, U-73343 (
Fig. 4A). Oocytes expressing the BK
Ca channel were incubated with U-73122 (1 µM) or U-73343 (1 µM) for 20 min. Treatment of LPA with U-73122 significantly attenuated the current from 332.50±26.41 to 41.43±32.95%. However, treatment of LPA with U-73343 did not show a significant difference (332.50±26.41 to 315.00±51.05%). These results demonstrate that the activation of the BK
Ca channel by LPA is also mediated by the PLC pathway.
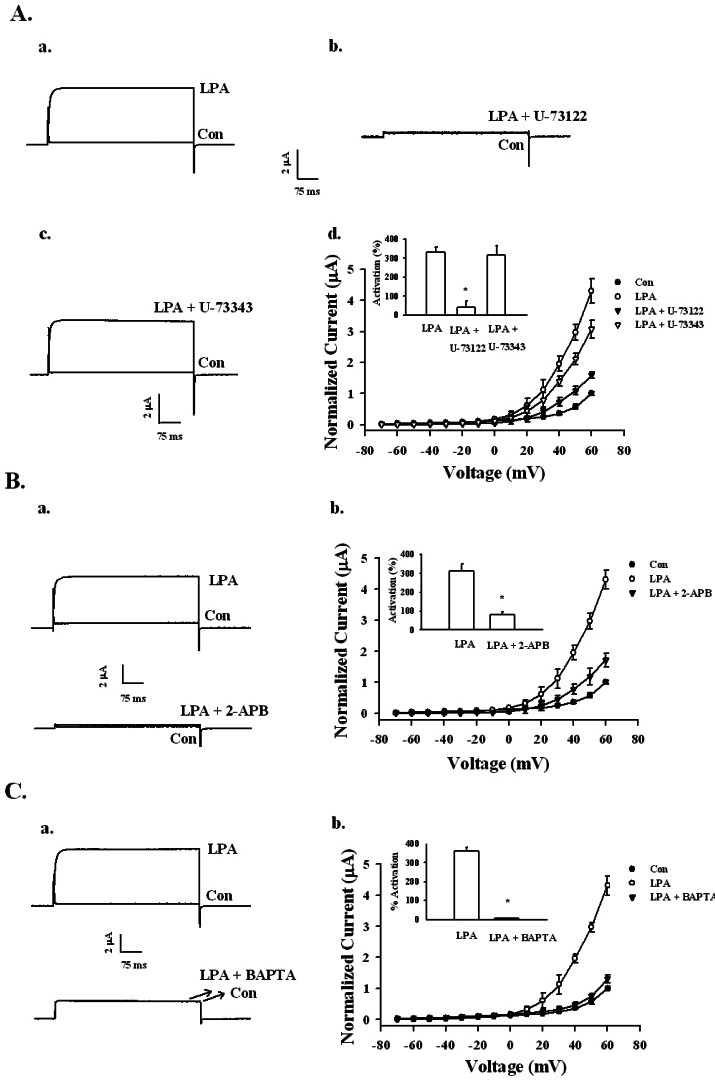 | Fig. 4Signaling pathway of LPA-mediated BKCa channel activation. (A) Effects of the PLC inhibitor on LPA-mediated BKCa channel activation. (a~c) A representative trace shows the activation of LPA-(100 nM) mediated currents in Xenopus oocytes expressing the BKCa channel in the absence or presence of inactive U73343 (1 µM) or active PLC inhibitor U73122 (1 µM). (d) Summary histograms of LPA-mediated activation on control, U-73122, or U-73343-treated groups (right lower panel). Data represent the mean±S.E.M. (n=10~11; *p<0.01). (B) Effect of IP3 receptor antagonist 2-APB on LPA-mediated (100 nM) BKCa channel activation. (a) Representative trace shows the activation of LPA-mediated (100 nM) currents in Xenopus oocytes expressing the BKCa channel in the absence or presence of 100 µM 2-APB for 20 min. (b) Summary histograms of LPA-mediated activation on control or 100 µM 2-APB (right lower panel). Data represent the mean±S.E.M. (n=12~14; *p<0.05). (C) Effect of intracellular Ca2+ chelator, BAPTA-AM, on LPA-mediated (100 nM) BKCa channel activation. (a) A representative trace shows the activation of LPA-mediated (100 nM) currents in Xenopus oocytes expressing the BKCa channel in the absence or presence of BAPTA-AM for 3 h. (b) Summary histograms of LPA-mediated activation on control or 100 µM BAPTA-AM (right lower panel). Data represent the mean±S.E.M. (n=10~12; *p<0.01). 
|
To study the relationship between the inositol 1,4,5-trisphosphate receptor and BK
Ca channel activation by LPA, we used 2-aminoethoxydiphenyl borate (2-APB), which blocks the inositol 1,4,5-trisphosphate (IP
3) receptor. The oocytes expressing the BK
Ca channel were pre-incubated with 100 µM 2-APB for 20 min before recording. In the absence of 2-APB, LPA (100 nM) activated the BK
Ca channel by 312.63±38.01%, whereas in the presence of 2-APB LPA (1 µM), the BK
Ca channel was activated by only 81.58±13.62% (
Fig. 4B). Thus, LPA-mediated activation of the BK
Ca channel involves the IP
3 receptor.
Next, we used BAPTA-AM (1,2-bis(
O-aminophenoxy) ethane-
N,
N,
N',
N'-tetraacetic acid), a cell permeable intracellular Ca
2+ chelator, to discover whether LPA-mediated activation of the BK
Ca channel was from elevated levels of free cytosolic Ca
2+. As shown in
Fig. 4C, pretreatment with BAPTA-AM (100 µM, 3 h) on oocytes significantly attenuated LPA-mediated BK
Ca channel activation. Thus, the LPA-mediated BK
Ca channel current after BAPTA-AM incubation was abolished. Therefore, LPA-mediated enhancement of BK
Ca channel currents is dependent on intracellular Ca
2+.
We also investigated the involvement of the PKC pathway in BK
Ca channel activation by LPA. Oocytes expressing the BK
Ca channel were incubated with 1.5 µM calphostin, an inhibitor of PKC, for 2 h. Calphostin also significantly attenuated LPA-mediated BK
Ca channel activation (
Fig. 5A). Thus, LPA-mediated BK
Ca channel activation includes the phospholipase C-IP
3 receptor-Ca
2+-protein kinase C pathways.
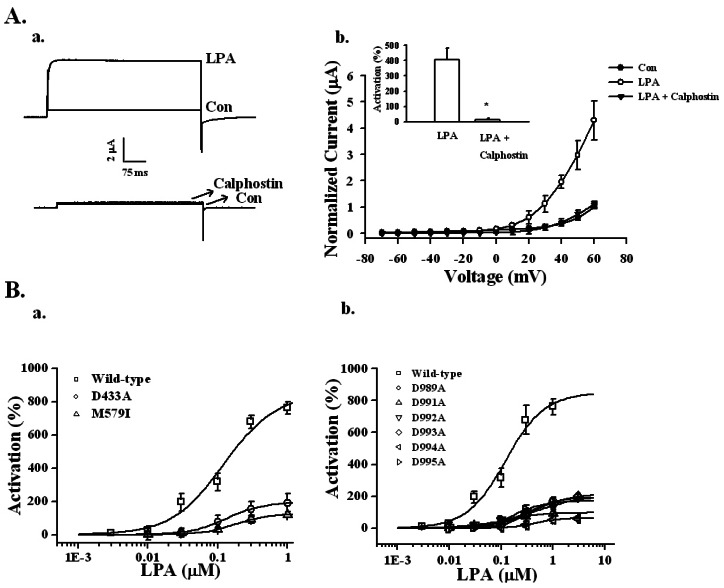 | Fig. 5Effects of PKC inhibitor and mutations in RCK1 and the Ca2+ bowl on LPA-mediated BKCa channel activation. (A-a) Effects of PKC inhibitor on LPA-mediated BKCa channel activation. A representative trace shows the activation of LPA-mediated (100 nM) currents in Xenopus oocytes expressing the BKCa channel in the absence or presence of 1.5 µM calphostin for 2 h. (A-b) Summary histograms of LPA-mediated BKCa channel activation in controls or with 1.5 µM calphostin (right lower panel). Data represent the mean±S.E.M. (n=10~12; *p<0.01). (B) Effect of mutations on the RCK1 and Ca2+ bowl domains in LPA-mediated BKCa channel activation. (B-a) The oocytes expressing wild-type or mutant BKCa channels in the RCK domain such as D433A or M579I were treated with LPA by a bathing application for 60 s. Mutations in the RCK domain attenuated the LPA concentration-responses (left panel). (B-b) The oocytes expressing wild-type or mutant BKCa channels in the Ca2+ bowl were treated with LPA by a bathing application for 60 s. Mutations in the Ca2+ bowl also attenuated LPA concentration-responses (right panel). Data represent the mean±S.E.M. (n=6). 
|
The BK
Ca channel contains two regulators of K
+ conductance (RCK) domains in the cytoplasmic domain [
3]. We constructed mutant BK
Ca channels in the RCK1 domain to decipher whether BK
Ca channel activation by LPA involves the RCK1 domain. For this, mutant BK
Ca channels with two RCK1s such as M579I and D433A were prepared [
22]. In wild-type, LPA (100 nM)-mediated BK
Ca channel activation was 316.5±75.10%. However, in the mutant M579I channel, LPA-mediated BK
Ca channel activation was significantly decreased by 30.5±14.12% (
Fig. 5Ba). In the mutant D433A channel, LPA-mediated (100 nM) BK
Ca channel activation was also significantly decreased by 77.5±38.62% (
Fig. 5Ba). The EC
50 values for the RCK1 mutants were 121.8±43.0, 134.3±7.9, and 177.2±16.3 nM for wild type, D433A, and M579I, respectively (
Fig. 5Ba).
In addition, we constructed six mutant BK
Ca channels at the Ca
2+ bowl, such as D989A, D991A, D992A, D993A, D994A, and D995A [
23]. The percentages of activation from 100 nM LPA were 317.50±60.90, 23.60±2.73, 41.20±5.49, 49.16±10.13, 28.14±6.16, 7.05±2.12, and 49.30±16.19% for wild-type, D989A, D991A, D992A, D993A, D994A, and D995A, respectively (n=9). In addition, the EC
50 values were 121.80±42.96, 334.22±13.20, 147.54±34.34, 434.53±120.27, 404.29±73.91, 411.30±76.58, and 183.46±3.23 nM for wild-type, D989A, D991A, D992A, D993A, D994A, and D995A, respectively (
Fig. 5Bb). These results showed that RCK1 and the Ca
2+ bowl are involved in LPA-mediated BK
Ca channel activation.
Go to :

DISCUSSION
In the present study, we investigated the signal couplings of LPA on BKCa channel activation by using a Xenopus oocyte gene expression system. Our results revealed three major findings. First, we observed that LPA treatment induced BKCa channel activation in concentration- and voltage-dependent manners via LPA receptor activation, while repeated treatments with LPA induced rapid desensitization. Second, the presence of PLC inhibitor, IP3 receptor antagonist, intracellular Ca2+ chelator, or PKC inhibitor greatly attenuated the LPA actions. Third, mutations in amino acid residues in the Ca2+ bowl and RCK domain greatly attenuated LPA-mediated enhancement of the BKCa channel currents. Thus, since BKCa channels play an important role in presynaptic nerve terminals and blood vessel smooth muscle cells, the findings in the present study suggest the possibility that LPA may be a novel BKCa channel regulator in the nervous and vascular systems via PLC-IP3-Ca2+ signal transduction pathways.
To confirm that the LPA-mediated [Ca
2+]
i transient is coupled to the activation of BK
Ca channels, we first treated oocytes with BAPTA-AM, a membrane permeable Ca
2+ chelator. As shown in
Fig. 4C, BAPTA-AM treatment abolished to 98.5% of the control (p<0.001, compared to BAPTA un-treatment). In addition, we constructed mutant channels in the Ca
2+ bowl and RCK domain. As shown in
Fig. 5B, mutations of several amino acid residues in the Ca
2+ bowl and RCK domain greatly attenuated LPA-mediated BK
Ca channel activation, indicating that extracellular LPA treatment induces the mobilization of cytosolic Ca
2+ from the endoplasmic reticulum; the increased Ca
2+ ions bind the Ca
2+ bowl and RCK domain in order to activate the BK
Ca channels.
BKCa channels are ideal pharmacological targets, since the channel opening increases the attenuation of neuron excitability and smooth muscle relaxation in an existing physiological system. The in vitro LPA enhancement of brain BKCa channel currents also has in vivo pharmacological effects. The enhancing effects of LPA on the channel currents may contribute to repolarization of the action potential neurons and vascular smooth muscle cells, while shortening the action potential duration. Thus, activating BKCa channels by LPA might be used to reduce over-excitability of the nervous system or to down-regulate hyperactivity of vascular smooth muscle cells. This study also raises the possibility that LPA participates in the regulation of synaptic transmission in nerve terminals and vascular systems.
On the other hand, recent report showed that LPA is also related with pain induction in spinal cord, since LPA synthesized in the spinal dorsal horn after nerve injury [
24]. LPA-mediated intracellular [Ca
2+]
i increase not only stimulates microglia to induce the BDNF synthesis through activation of P
2X4 receptors, but also activates activation of microglial BK
Ca channels [
25]. BDNF secreted from microglia in turn further enhance the activities of BK channels [
19,
26]. Thus, it seems that the physiological or pharmacological roles of LPA-mediated BK channel activations might be dependent on cell types or organs. Further investigations into the potential clinical applications of LPA on the nervous and vascular systems are required.
In conclusion, we found that activation of Gαq/11 protein-coupled LPA receptor by LPA is coupled to BKCa channel activation and that Ca2+ bowl and RCK domain of BKCa channel also play important roles in LPA-mediated BKCa channel activation using site-directed mutagenesis method. The present findings may provide a molecular basis for LPA effects on the nervous and vascular systems.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download