INTRODUCTION
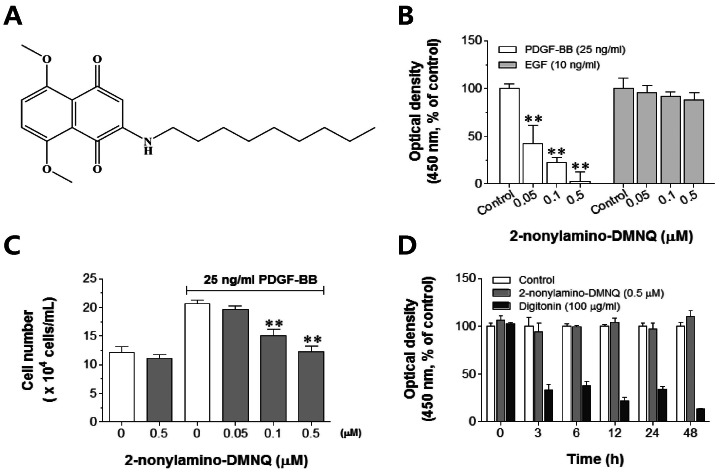 | Fig. 1Effects of 2-nonylamino-DMNQ on VSMC proliferation and viability. (A) The chemical structure of 2-nonylamino-DMNQ. (B) The ability of 2-nonylamino-DMNQ to regulate both PDGF- and EFG-induced VSMC proliferation. VSMCs cultured in serum-free medium were incubated with various concentrations of 2-nonylamino-DMNQ (0.05~0.5 µM) for 24 h and then stimulated with PDGF-BB (25 ng/ml) or EGF (10 ng/ml). The optical density at 450 nm was determined using a microplate reader. (C) The ability of 2-nonylamino-DMNQ to regulate cell counts. VSMCs cultured in serum-free medium were treated with various concentrations of 2-nonylamino-DMNQ for a further 24 h, stimulated with PDGF-BB (25 ng/ml), and counted using a hemocytometer. (D) Effects of 2-nonylamino-DMNQ on cell viability. VSMCs cultured in serum-free medium were incubated with control (DMSO, 1%), or 2-nonylamino-DMNQ (0.5 µM) or digitonin (100 µg/ml) for the indicated periods, and optical densities at 450 nm were measured. Values are expressed as means±S.E.M. of four similar and independent experiments. **p<0.01, statistically significant differences compared to PDGF control (PDGF-BB-stimulated, but no 2-nonylamino-DMNQ) or EGF control (EGF-stimulated, but no 2-nonylamino-DMNQ). |
METHODS
Materials
Cell proliferation and viability assays
[3H]-Thymidine incorporation assay
Analysis of cell cycle progression
Western blotting
Statistical analysis
RESULTS
Effects of 2-nonylamino-DMNQ on VSMC proliferation and viability
Effects of 2-nonylamino-DMNQ on DNA synthesis and cell cycle progression
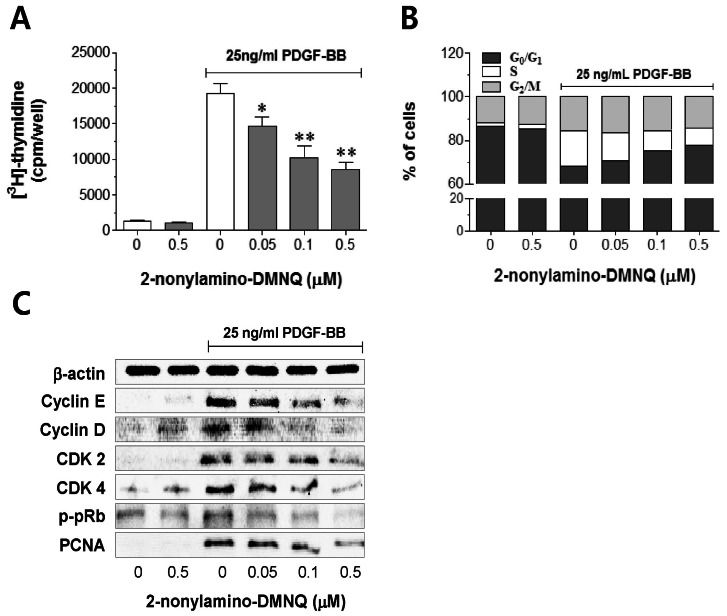 | Fig. 2Effects of 2-nonylamino-DMNQ on [3H]-thymidine incorporation, cell cycle progression, and cell cycle regulatory proteins in PDGF-stimulated VSMCs. (A) The ability of 2-nonylamino-DMNQ to regulate [3H]-thymidine uptake. PDGF-BB (25 ng/ml)-stimulated VSMCs were treated with or without the indicated concentrations of 2-nonylamino-DMNQ for 24 h, and then incubated with [3H]-thymidine (2 µCi/ml) for 4 h. Radioactivity was determined using a liquid scintillation counter. Data are representative of four independent experiments, and are expressed as means±S.E.M. *p<0.05, **p<0.01, statistically significant differences compared to PDGF control (PDGF-BB-stimulated, but no 2-nonylamino-DMNQ). (B) The ability of 2-nonylamino-DMNQ to regulate cell cycle progression. VSMCs in serum-free medium were incubated with or without 2-nonylamino-DMNQ (0.05~0.5 µM) for 24 h, stimulated with 25 ng/ml PDGF-BB for 24 h, harvested, and subjected to flow cytometric analysis of DNA content as described in Methods. Each point was derived from a representative experiment, in which data from at least 11,000 events were obtained. The results are representative of three similar and independent experiments. (C) The ability of 2-nonylamino-DMNQ to regulate cell cycle regulatory proteins. Serum-starved VSMCs were pretreated with or without the indicated concentrations of 2-nonylamino-DMNQ for 24 h, stimulated with PDGF-BB (25 ng/ml) for a further 24 h, and the expression of cyclin E, cyclin D CDK2, CDK4, and PCNA, and the phosphorylation of pRb were measured as described in Methods. Total β-actin was used for normalization. Images are representative blots of four similar and independent experiments. |
Effects of 2-nonylamino-DMNQ on the activation of PDGF receptor tyrosine kinase
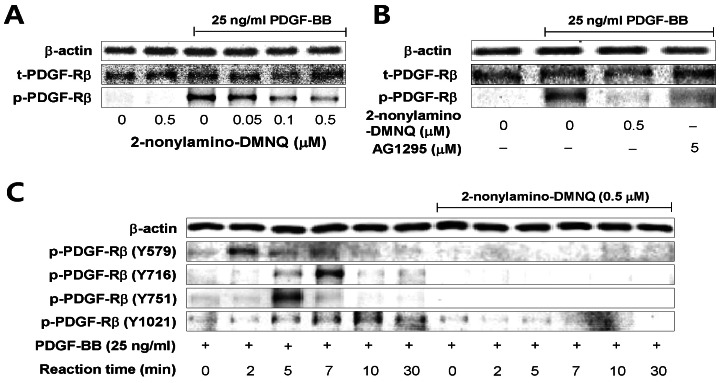 | Fig. 3Effects of 2-nonylamino-DMNQ on PDGF-induced PDGF-Rβ activation in VSMCs. (A) The ability of 2-nonylamino-DMNQ to inhibit PDGF-Rβ activation induced by PDGF. Serum-starved VSMCs were treated with or without the indicated concentrations of 2-nonylamino-DMNQ for 24 h, stimulated with PDGF-BB (25 ng/ml) for 3 min, and the phosphorylation of PDGF-Rβ was measured as described in Methods. (B) Comparison of the ability of 2-nonylamino-DMNQ and AG1295 to inhibit PDGF-induced PDGF-Rβ activation. VSMCs cultured in serum-free medium were incubated with or without the indicated concentrations of 2-nonylamino-DMNQ or AG1295, a selective inhibitor of PDGF receptor kinase, for 24 h, stimulated with PDGF-BB (25 ng/ml) for 3 min, and the phosphorylation of PDGF-Rβ was measured as described in Methods. (C) The ability of 2-nonylamino-DMNQ to regulate phosphorylation at four different tyrosine residues in PDGF-stimulated PDGF-Rβ. Serum-starved VSMCs were incubated with or without 0.5 µM 2-nonylamino-DMNQ for 24 h. After reacting VSMCs with PDGF-BB (25 ng/ml) for the indicated periods, the phosphorylation of PDGF-Rβ at Tyr579, Tyr716, Tyr751, and Tyr1021 residues was measured as described in Methods. Images are representative blots of three similar and independent experiments. |
Effects of 2-nonylamino-DMNQ on the phosphorylation of STAT3, ERK1/2, Akt, and PLCγ
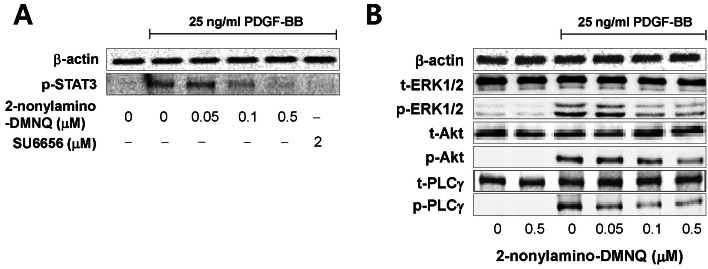 | Fig. 4Effects of 2-nonylamino-DMNQ on STAT3, ERK1/2, Akt and PLCγ1 phosphorylation in PDGF-stimulated VSMCs. (A) The ability of 2-nonylamino-DMNQ to regulate STAT3 activation. VSMCs cultured in serum-free medium were incubated with or without the indicated concentrations of 2-nonylamino-DMNQ or SU6656, a Src family kinase inhibitor, for 24 h, stimulated with 25 ng/ml PDGF-BB for 10 min, and the phosphorylation of STAT3 was measured as described in Methods. Images are representative blots of four similar and independent experiments. (B) The ability of 2-nonylamino-DMNQ to regulate ERK1/2, Akt, and PLCγ1 activation. Serum-starved VSMCs were treated with or without the indicated concentrations of 2-nonylamino-DMNQ for 24 h, stimulated with 25 ng/ml PDGF-BB for 5 min (ERK1/2 and PLCγ1) or 15 min (Akt), and the ERK1/2, Akt and PLCγ1 phosphorylation were measured as described in Methods. Images are representative blots of four similar and independent experiments. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download