Abstract
In the present study, the effect of intrathecal (i.t.) or intracerebroventricular (i.c.v.) administration with cholera toxin (CTX) on the blood glucose level was examined in ICR mice. The i.t. treatment with CTX alone for 24 h dose-dependently increased the blood glucose level. However, i.c.v. treatment with CTX for 24 h did not affect the blood glucose level. When mice were orally fed with D-glucose (2 g/kg), the blood glucose level reached to a maximum level at 30 min and almost returned to the control level at 120 min after D-glucose feeding. I.c.v. pretreatment with CTX increased the blood glucose level in a potentiative manner, whereas i.t. pretreatment with CTX increased the blood glucose level in an additive manner in a D-glucose fed group. In addition, the blood glucose level was increased in formalin-induced pain animal model. I.c.v. pretreatment with CTX enhanced the blood glucose level in a potentiative manner in formalin-induced pain animal model. On the other hand, i.t. pretreatment with CTX increased the blood glucose level in an additive manner in formalin-induced pain animal model. Our results suggest that CTX administered supraspinally or spinally differentially modulates the regulation of the blood glucose level in D-glucose fed model as well as in formalin-induced pain model.
The experience of pain in response to noxious stimuli serves a crucial biological purpose: it alerts a living organism to environmental dangers, inducing counteractive responses against further damage [1]. Pain is a multi-dimensional process involving the physical, emotional and perceptual integration of noxious information. The physical component is relayed via the spinal cord to several brain areas to initiate the detection of pain. The emotional aspect is encoded by the limbic system and encapsulates the relationship between pain and mood [2].
Cholera toxin (CTX), an exotoxin produced by vibrio cholerae, is composed of a toxic A subunit covalently linked to a pentamer of B subunits. The A subunit is an enzymatic component and ADP-ribosylates the GTP-bound form of Gαs subunit which dissociates from βγ subunits, thereby activating adenylyl cyclase [3]. The increase of intracellular cAMP causes protein kinase A (PKA) activation which, in turn, phosphorylates a cAMP response element binding protein (CREB) [4], or opening the cyclic-nucleotide-gated ion channels which evoke the depolarization [5]. The non-toxic B subunit binds to a cell-surface ganglioside, GM1, and facilitates entry of the toxin into the cell. In addition B subunit is also known to affect cell growth and differentiation [6]. CTX-sensitive G-proteins appear to be involved in the regulation of nociception as well as antinociception. For example, we found previously that i.t. pretreatment with CTX attenuates pain response induced by i.t. injection with glutamate, NMDA, AMPA and kainic acid [7]. Furthermore, supraspinally or spinally administration with CTX show the modulatory role in the regulation of antinociception induced by various opioids [8].
Recently, we have reported that various types of pain stimulation cause an elevation of the blood glucose level and differentially modulates the blood glucose level in D-glucose-fed model [9]. In addition, spinal administration with CTX differentially affects pain behaviors induced by various pro-inflammatory cytokines [10]. Although the blood glucose regulations in pain and D-glucose-fed models have been well studied, the roles of CTX-sensitive G-proteins located at the brain and the spinal cord in the regulation of blood glucose level has not been well characterized yet. Thus, in the present study, the effect of i.t. or i.c.v. administration with CTX on the blood glucose level was examined. Furthermore, the possible modulatory roles of CTX-sensitive G-proteins located at the brain as well as the spinal cord in the regulation of blood glucose level in D-glucose-fed and pain animal models were examined.
The experiments were approved by the Hallym University Animal Care and Use Committee (Registration Number: Hallym 2009-05-01). All procedures were conducted in accordance with the 'Guide for Care and Use of Laboratory Animals' published by the National Institutes of Health and the ethical guidelines of the International Association for the Study of Pain.
Male ICR mice (MJ Co., Seoul, Korea) weighing 20~25 g were used for all the experiments. Animals were housed 5 per cage in a room maintained at 22±0.5℃ with an alternating 12 h light-dark cycle. Food and water were available ad libitum. The animals were allowed to adapt to the laboratory for at least 2 h before testing and were only used once. Experiments were performed during the light phase of the cycle (10:00~17:00). The animals were fasted for 16 h. The behavioral scores were verified by repeat trials, and the observers of pain-induced behavior were blind to drug treatments.
Oral administration was performed with gavage in a volume of 1 ml/kg body weight. Mice were fasted overnight (16 h) and D-glucose (2 g/kg body weight) administered orally once. The blood glucose level was measured at 0, 30, 60, and 120 min after D-glucose administration.
I.t. administration was performed in conscious mice following the method of Hylden using a 30-gauge needle connected to a 25µl Hamilton syringe with polyethylene tubing [11]. The i.t. injection volume was 5µl and the injection site was verified by injecting a similar volume of 1% methylene blue solution and determining the distribution of the injected dye in the spinal cord. The dye injected i.t. was distributed both rostrally and caudally but with short distance (about 0.5 cm) and no dye was found in the brain. The success rate for the injections was consistently found to be over 95%, before the experiments were done. The i.c.v. administration followed the method described by Haley [12]. Each mouse was grasped firmly without anesthesia by the loose skin behind the head. The skin was pulled taut. A 30-guage needle attached to a 25µl syringe was inserted perpendicularly through the skull into the brain and solution was injected. The injection site was 2 mm from either side of the midline on a line drawn through the anterior base of the ears. The i.c.v. injection volumes were 5µl, and the injection sites were verified by injecting a similar volume of 1% methylene blue solution and determining the distribution of the injected dye in the ventricular space. The success rate for prior injections with this technique was over 95%.
This test, previously published by Hunskaar was carried out in mice [13]. Ten microliters of 1.0% formalin solution, made up in physiologic saline (0.9% NaCl), was injected subcutaneously (s.c.) under the plantar surface of the left hind paw. Control animals for the formalin nociceptive test received a similar volume of physiologic saline into the left hind paw. The mice were administered with s.c. formalin and then blood glucose level was measured at 30, 60, and 120 min after administration. The number of animals used for each group was 8~10.
The blood glucose level was measured at 24 h after i.t. or i.c.v. injection with CTX (n=8~10). The blood was collected shortly as much as possible with a minimum volume (1µl) from the tail-vein. The glucose level was measured using Accu-Chek Performa blood glucose monitoring system (glucometer) (Mannheim, Baden-Württemberg, Germany).
Formalin and D-glucose were purchased from Sigma Chemical Co. (St. Louis, MO, USA). CTX was purchased from Tocris Bioscience Co. (Minneapolis, MN, USA). All drugs were prepared just before use. Blood glucose meter, lancing device and strips were purchased from Roche Diagnostics (Accu-Chek Performa, Germany).
Statistical analysis was carried out by student t test GraphPad Prism Version 4.0 for Windows (GraphPad Software, San Diego, CA, USA). The area under the curve (AUC) was analyzed by one-way ANOVA. p values less than 0.05 were considered to indicate statistical significance. All values were expressed as the mean±S.E.M. In our study, we established the mean blood glucose value of the control group through many experiments under matching conditions. Selected mice of established blood glucose level were then used in replication experiments.
Mice were treated with various doses (0.05~0.5µg/5µl) of i.t. or i.c.v with CTX. The blood glucose level was measured 24 h after CTX treatment. As shown in Fig. 1A, the blood glucose level was significantly and dose-dependently increased after i.t. administration with CTX. However, i.c.v. treatment with CTX did not affect the blood glucose level as shown in Fig. 1B.
Effect of i.t. or i.c.v. treatment with CTX on the blood glucose level in D-glucose-fed model was examined. As shown in Fig. 2A and 2B, the blood glucose level reached to a maximum level at 30 min and returned to the basal level at 2 h after mice were fed orally with D-glucose (2 g/kg). I.t. pretreatment with CTX increased the blood glucose level in an additive manner when mice were fed orally with D-glucose (Fig. 2A and 2C). The blood glucose levels were enhanced in or i.c.v. treatment with CTX group in a potentiative manner when mice were fed orally with D-glucose as shown in Fig. 2B and 2D.
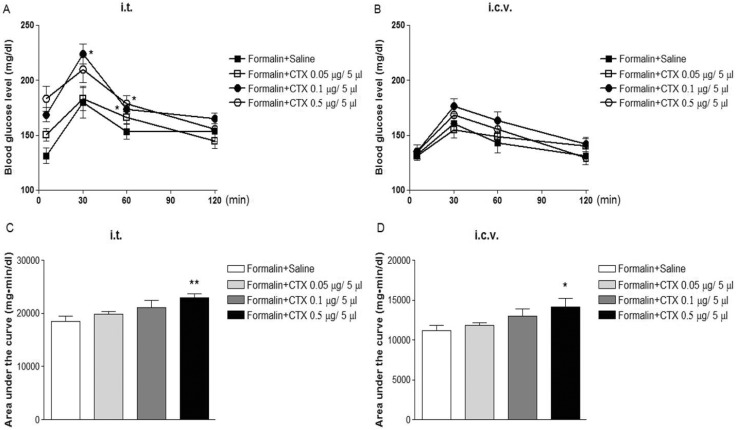
Effect of i.t. or i.c.v. treatment with CTX on the blood glucose level induced by formalin administration was examined. I.t. pretreatment with CTX increased formal-in-induced increase in blood glucose level in an additive manner (Fig. 3A and 3C). However, the blood glucose level induced by formalin administration were enhanced in a potentiative manner when mice were pretreated with i.c.v. with CTX as shown in Fig. 3B and 3D.
We found for the first time that spinally administered with CTX increases the blood glucose level. Spinally administered CTX-induced response was dose-dependent. Although the exact mechanism of spinally administered CTX-induced increase in blood glucose level is not known yet, the results of the present study suggest that CTX-sensitive G-proteins located at the spinal cord appear to be involved in the regulation of the blood glucose level. However, CTX-sensitive G-proteins located at the supraspinal brain sites may not responsible for the regulation of the blood glucose level.
When mice were fed orally with D-glucose, the blood glucose level was increased. Our result is in line with the previous studies which show D-glucose feeding increases the blood glucose level [9,14]. Although supraspinal treatment with CTX alone does not affect the blood glucose level (Fig. 1B), we found in the present study that supraspinally pretreated with CTX further enhances the blood glucose level in a potentiative manner in D-glucose fed group (Fig. 2D). Thus, the current findings suggest that CTX-sensitive G-proteins located at the supraspinal brain sites may exert a modulatory role in the regulation of the blood regulation after the D-glucose was absorbed into the blood stream. However, the exact mechanisms and locations of the brain sites should be further examined in the future study. In contrast to the results with supraspinally-treated CTX group, spinal treatment with CTX increases the blood glucose level in an additive manner in the group of mice fed orally with D-glucose. Thus, it is speculated that CTX-sensitive G-proteins located at the spinal cord appear not to be involved in the modulatory regulation of the blood glucose level in the group of mice fed with D-glucose, although CTX-sensitive G-proteins alone located at the spinal cord may play an important role for the regulation of blood glucose level.
We found also in the present study that the blood glucose level is increased by formalin-induced pain stimulation (Fig. 3A and 3B). We recently found that the blood glucose level is elevated in acetic acid-induced writhing pain animal model as well as formalin pain model [9]. Furthermore, we recently found that pain stimulation induced by proinflammatory cytokines such as TNF-α (tumor necrosis factor-α), IFN-γ (interferon-γ) and IL-1β (inetrleukin1β) administered spinally, also causes an elevation of the blood glucose level [9,14]. Similar to the results found in D-glucose fed animal model, we found in the present study that supraspina pretreatment with CTX further enhances the blood glucose level induced by formalin-induced pain stimulation (Fig. 3D). On the other hand, spinal treatment with CTX increases the blood glucose level induced by formalin-induced pain stimulation only in an additive manner (Fig. 3A). Our results suggest that CTX administered supraspinally and spinally appears to regulate the blood glucose level in a differential manner. It is suggested that CTX-sensitive G-proteins located at the supraspinal brain sites, but not in the spinal cord, may exert a modulatory role in the regulation of the blood glucose level induced by formalin-induced pain stimulation.
The findings of the present study further demonstrate our previous findings that the spinal cord is an important site for the regulation of the blood glucose level [14,15]. In earlier study, Sala et al. [16] have reported that less dose of insulin is used to regulate the blood glucose level in patients with spinal cord injury. In addition, we found recently that substance P and other types of pro-inflammatory cytokines such as TNF-α, IL-1β and IFN-γ administered spinally causes an elevation of the blood glucose level [9,14]. Studies on the detailed role of the spinal cord in the regulation of the blood glucose level should be further investigated in the future study.
ACKNOWLEDGEMENTS
This research was supported by Priority Research Centers (2012-R1A6A1048184) and Basic Science Research (2012-0001569) Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, and Hallym University Research Fund (HRF-201207-004).
References
1. de los Santos-Arteaga M, Sierra-Domínguez SA, Fontanella GH, Delgado-García JM, Carrión AM. Analgesia induced by dietary restriction is mediated by the kappa-opioid system. J Neurosci. 2003; 23:11120–11126. PMID: 14657170.
2. Blackburn-Munro G, Blackburn-Munro R. Pain in the brain: are hormones to blame? Trends Endocrinol Metab. 2003; 14:20–27. PMID: 12475608.

3. Won JS, Suh HW. The comparative analysis of proenkephalin mRNA expression induced by cholera toxin and pertussis toxin in primary cultured rat cortical astrocytes. Brain Res Mol Brain Res. 2001; 88:83–93. PMID: 11295234.

4. Bullock BP, Habener JF. Phosphorylation of the cAMP response element binding protein CREB by cAMP-dependent protein kinase A and glycogen synthase kinase-3 alters DNA-binding affinity, conformation, and increases net charge. Biochemistry. 1998; 37:3795–3809. PMID: 9521699.

5. Kaupp UB. The cyclic nucleotide-gated channels of vertebrate photoreceptors and olfactory epithelium. Trends Neurosci. 1991; 14:150–157. PMID: 1710853.
6. Facci L, Skaper SD, Favaron M, Leon A. A role for gangliosides in astroglial cell differentiation in vitro. J Cell Biol. 1988; 106:821–828. PMID: 2831235.
7. Chung KM, Lee KC, Song DK, Huh SO, Choi MR, Kim YH, Suh HW. Differential modulatory roles of cholera toxin and pertussis toxin in the regulation of pain responses induced by excitatory amino acids administered intrathecally in mice. Brain Res. 2000; 867:246–249. PMID: 10837821.

8. Suh HW, Song DK, Sim YB, Choi YS, Kim YH. Effect of intrathecal or intracerebroventricular pretreatment with cholera toxin on antinociception induced by opioids administered intracerebroventricularly in mice. Pharmacol Commun. 1995; 6:187–294.
9. Sim YB, Park SH, Kang YJ, Jung JS, Ryu OH, Choi MG, Suh HW. Various pain stimulations cause an increase of the blood glucose level. Animal Cells Syst. 2012; 16:385–390.

10. Kwon MS, Shim EJ, Seo YJ, Choi SS, Lee JY, Lee HK, Suh HW. Differential modulatory effects of cholera toxin and pertussis toxin on pain behavior induced by TNF-alpha, interleukin-1beta and interferon-gamma injected intrathecally. Arch Pharm Res. 2005; 28:582–586. PMID: 15974446.
11. Hylden JL, Wilcox GL. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981; 217:212–215. PMID: 6167328.

12. Haley TJ. Pharmacological effects produced by intracerebral administration of drugs of unrelated structure to conscious mice. Arch Int Pharmacodyn Ther. 1957; 110:239–244. PMID: 13435953.
13. Hunskaar S, Berge OG, Hole K. Antinociceptive effects of orphenadrine citrate in mice. Eur J Pharmacol. 1985; 111:221–226. PMID: 4018126.

14. Sim YB, Park SH, Kang YJ, Jung JS, Ryu OH, Choi MG, Suh HW. Interleukin-1β (IL-1β) increases pain behavior and the blood glucose level: possible involvement of sympathetic nervous system. Pharmacol Biochem Behav. 2012; 102:170–176. PMID: 22548833.

15. Sim YB, Park SH, Kang YJ, Kim SS, Kim CH, Kim SJ, Jung JS, Ryu OH, Choi MG, Suh HW. Central anti-diabetic action of biguanide and thizolidinediones in D-glucose fed and streptozotocin-treated mouse models. Neurosci Lett. 2012; 528:73–77. PMID: 22960361.

16. Sala F, Menna G, Bricolo A, Young W. Role of glycemia in acute spinal cord injury Data from a rat experimental model and clinical experience. Ann N Y Acad Sci. 1999; 890:133–154. PMID: 10668421.

Fig. 1
Effect of i.t. or i.c.v. administration with CTX on the blood glucose level. Various doses of CTX (from 0.05 to 0.5µg/5µl) were pretreated i.t. (A) or i.c.v. (B) for 24 h. The blood glucose level was measured after 24 h after CTX pretreatment. The vertical bars indicate the standard error of mean (**p<0.01, ***p<0.001; compared to saline group).
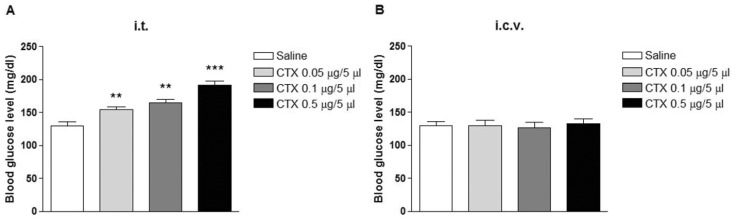
Fig. 2
Effect of i.t. or i.c.v. administration with CTX on the blood glucose level in D-glucose fed model. Mice were pretreated either i.t. (A) or i.c.v. (B) with CTX (from 0.05 to 0.5µg/5µl) for 24 h. The blood glucose level was measured at 30, 60, and 120 min after either saline or D-glucose (2 g/kg) was fed orally. The data were analyzed by area under the curve (C, D). The vertical bars indicate the standard error of mean (*p<0.05, **p<0.01, ***p<0.001; compared D-Glucose (D.G)+saline group).
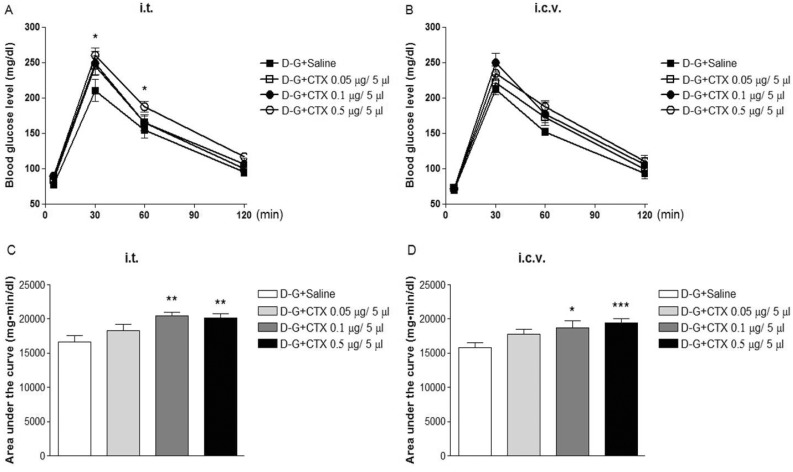
Fig. 3
Effect of i.t. or i.c.v. administration with CTX on the blood glucose level in formalin-induced pain model. Mice were pretreated either i.t. (A) or i.c.v. (B) with CTX (0.5µg/5µl) for 24 h. And then, mice were treated with formalin into the plantar of the hindpaw. The blood glucose level was measured at 30, 60, and 120 min right after the formalin injection. The data were analyzed by area under the curve (C, D). The vertical bars indicate the standard error of mean (*p<0.05, **p<0.01; compared+saline group).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download