INTRODUCTION
METHODS
Materials
Preparation of Xenopus oocytes and microinjection
cRNA preparation of the GABAC receptor ρ 1 subunit
Data recording
Data analysis
RESULTS
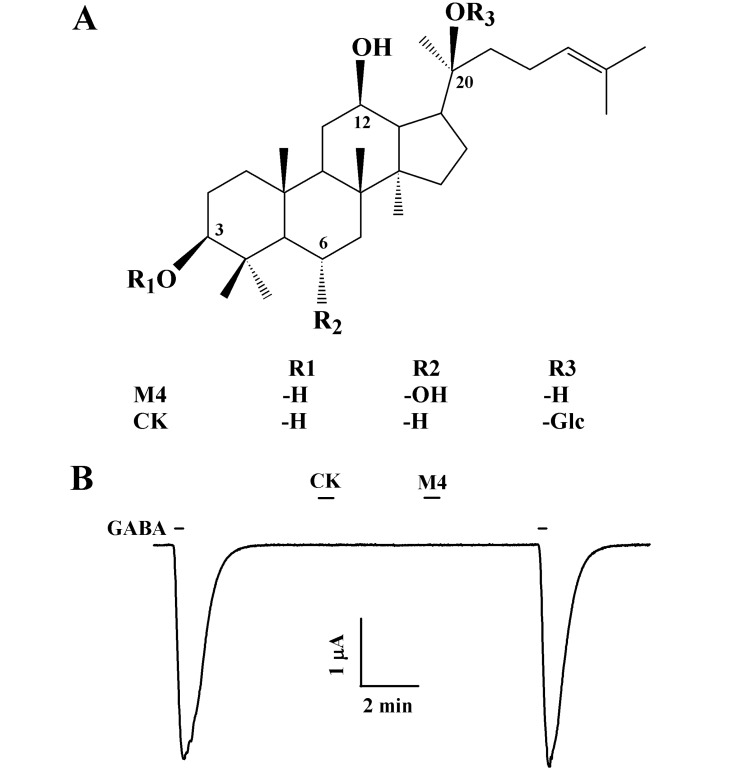
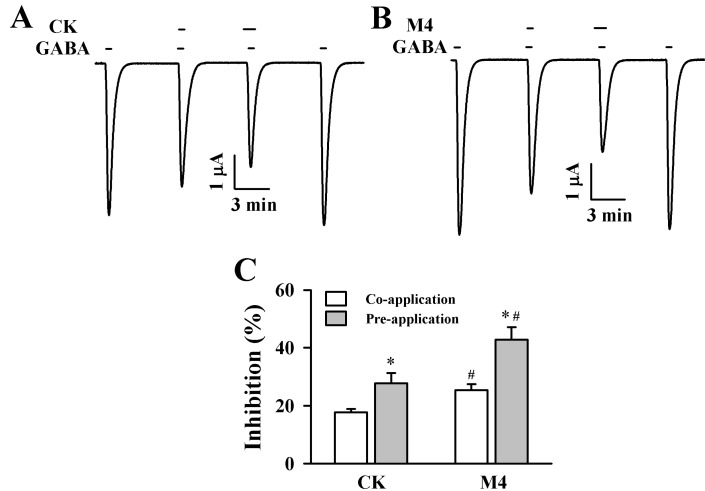 | Fig. 2Effect of CK and M4 on IGABA in oocytes expressing the ρ1 GABAC receptor. (A) GABA (2 µM) was first applied and then was co- or pre-treated with CK (100 µM). Co-treatment of CK with GABA and pre-treatment of CK before GABA application inhibited IGABA in oocytes expressing ρ1 GABAC receptors. (B) GABA (2 µM) was first applied and then co- or pre-treated with M4 (100 µM). Co-treatment of M4 with GABA and pre-treatment of M4 before GABA application inhibited IGABA in oocytes expressing ρ1 GABAC receptors. The resting membrane potential of oocytes was approximately -35 mV and oocytes were voltage clamped at a holding potential of -80 mV prior to drug application. Traces are representative of 8~12 separate oocytes from 3 different frogs. (C) Summary of percent inhibition by CK and M4 of IGABA calculated from the average of the peak inward current elicited by GABA alone before CK and M4 and the peak inward current elicited by GABA alone after co- and pre-treatment of CK and M4 with GABA. Each point represents the mean±S.E.M. (n=9~12 from 3 different frogs). *p <0.05 as compared to co-treatment of CK and M4, and #p<0.05 as compared to CK. |
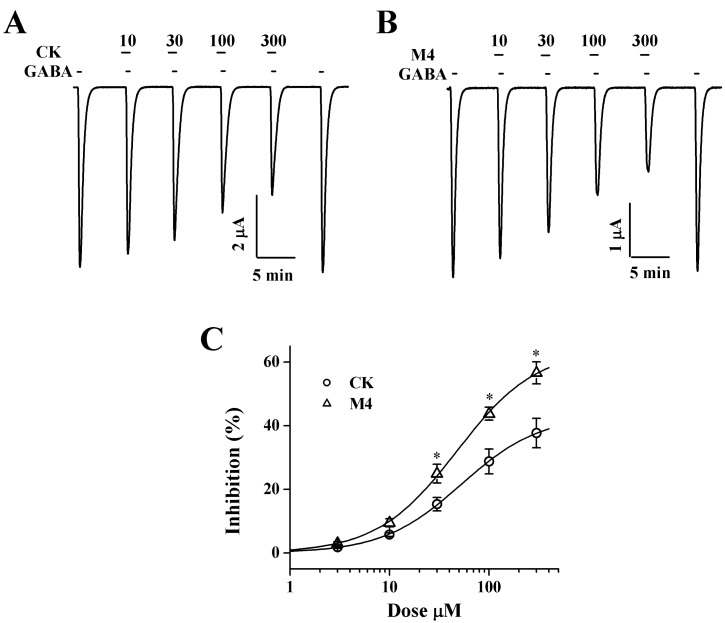 | Fig. 3Concentration-dependent effects of CK and M4 on IGABA in oocytes expressing ρ1 GABAC receptors. (A, B) The trace shows that CK and M4 inhibited the currents elicited by GABA (GABA; 2 µM) in a dose-dependent manner. (C) Percent inhibition by CK and M4 of IGABA was calculated from the average of the peak inward current elicited by GABA alone before CK and M4 and the peak inward current elicited by GABA alone after pre-treatment of CK and M4 before GABA. The continuous line shows the curve fitted according to the equation. Each point represents the mean±S.E.M. (n=9~12 from 3 different frogs). *p<0.005 as compared to CK. |
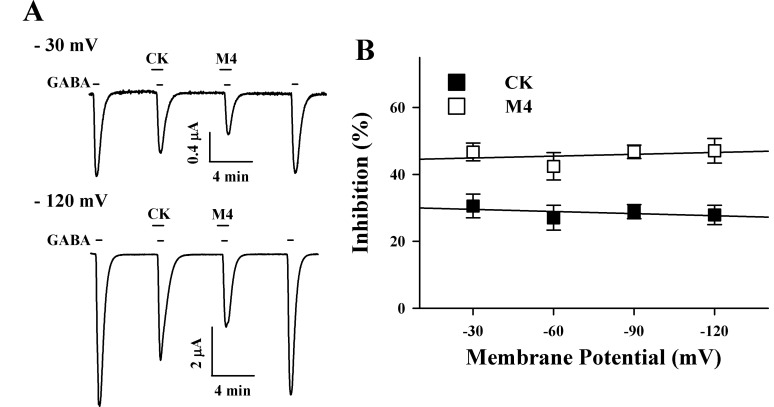 | Fig. 4Voltage-independent inhibition by CK and M4. (A) GABA (2 µM) was first applied and pre-treatment of CK (100 µM) or M4 (100 µM) before GABA application inhibited IGABA in oocytes expressing ρ1 GABAC receptors. The inhibitory degrees were very similar at -30 mV and -120 mV of membrane potential. (B) Voltage-independent inhibition of IGABA in GABAC receptors by CK and M4. Values were obtained from the receptors in the absence or presence of 100 µM CK and M4 at the indicated membrane holding potentials. The observed effects of CK and M4 were correlated with membrane potential. No significant effects were noted for CK (r2=0.28, p>0.31) and M4 (r2=0.10, p>0.44). |
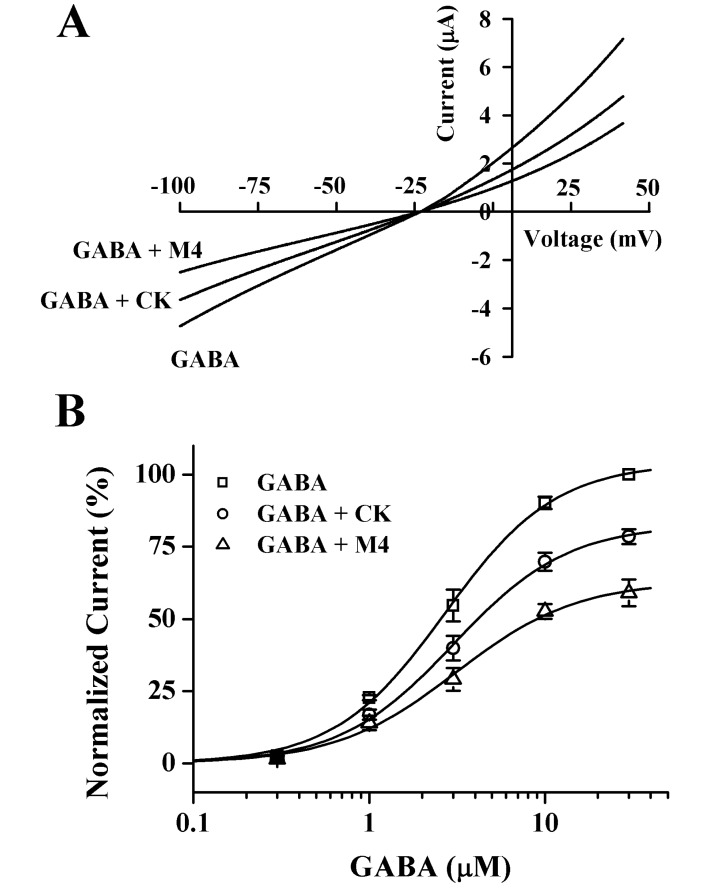 | Fig. 5Current-voltage relationships and concentration-dependent effects of GABA on CK- and M4-mediated inhibition of IGABA. (A) Current-voltage relationships of IGABA inhibition by CK and M4 in GABAC receptors. Representative current-voltage relationships were obtained using voltage ramps of -100 to +40 mV for 300 ms at a holding potential of -80 mV. Voltage steps were applied before and after application of 2 µM GABA in the absence or presence of 100 µM CK and M4. (B) Concentration-response relationships for GABA in GABAC receptors with GABA applied (0.3~30 µM) alone or with GABA plus pre-treatment of 100 µM CK and M4 before GABA application. IGABA of oocytes expressing the GABAC receptors was measured using the indicated concentration of GABA in the absence (□) or presence (○) of 100 µM CK or presence (△) of 100 µM M4. Oocytes were voltage-clamped at a holding potential of -80 mV. Each point represents the mean±S.E.M. (n=8~12/group). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download