INTRODUCTION
METHODS
Animal preparation and drug administration
Immunohistochemistry for c-Fos
Measurement of arterial blood pressure and heart rate
Statistical analysis
RESULTS
Ginsenosides reduce the number of Fos-IR neurons increased by NS to tooth in the trigeminal spinal subnuclei and thalamus
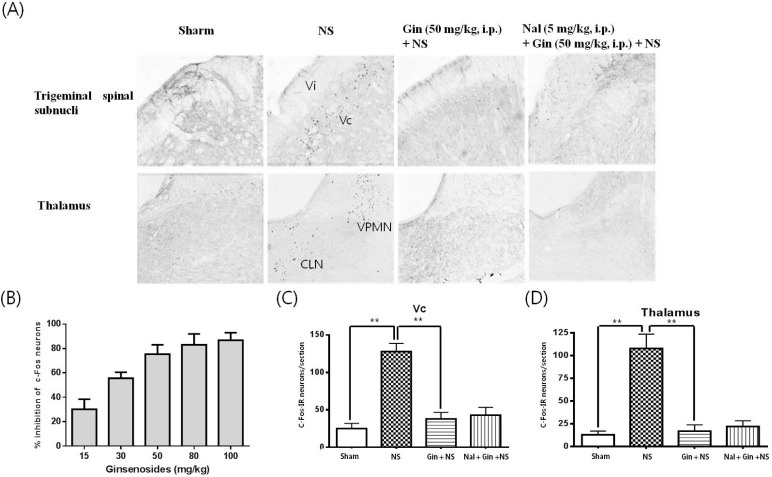 | Fig. 2Effects of ginsenosides on the number Fos-IR neurons increased by NS to tooth in the trigeminal spinal subnuclei and thalamus. (A) c-Fos immunoreactivity in trigeminal spinal subnuclei and thalamus. (B~D) Fos-IR neurons were increased in the trigeminal spinal subnucleus candalis (Vc) and transitional region between subnucleus interpolaris (Vi), thalamic ventral posteromedial nucleus (VPM) and centrolateral nucleus (CLN). 1.5 h after NS to the upper and lower incisor pulp with 2 M KCl. Ginsenosides reduced the number of Fos-IR neurons increased by NS to tooth in a dose-dependent manner and IC75 for ginsenosides is 50 mg/kg. Naloxon (Nal; 5 mg/kg, i.p.), an opioid antagonist, did not block the inhibitory effects of ginsenosides on the number of Fos-IR neurons increased by NS to tooth. Data represent as mean±SEM (n=5), **p<0.01. |
Ginsenosides rescue the cardiovascular responses elevated by NS to tooth
 | Fig. 3Effects of ginsenosides on the cardiovascular responses elevated by NS to tooth. The blood pressure (BP) and heart rate (HR) were increased immediately after NS to tooth. Ginsenosides (Gin; 50 mg/kg, i.p.) ameliorated the BP and HR increased by NS to tooth. Data represent as mean±SEM (n=12), *p<0.05. |
Ginsenosides decrease the number of Fos-IR neurons increased by NS to tooth in central cardiovascular regulating areas
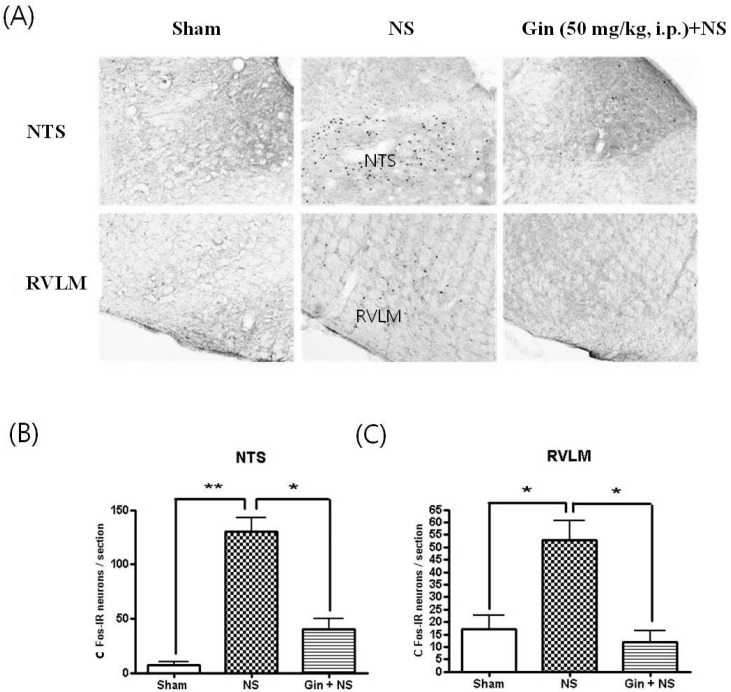 | Fig. 4Effects of ginsenosides on the number of Fos-IR neurons increased by NS to tooth in the nucleus tractus solitaries (NTS) and rostral ventrolateral medulla (RVLM). (A) c-Fos immunoreactivity in NTS and RVLM. (B, C) The NS to tooth caused a robust increment of Fos-IR neurons in the NTS and RVLM, central pressor regulating areas and ginsenosides (Gin; 50 mg/kg, i.p.) reduced the number of Fos-IR neurons increased by NS to tooth in the NTS and RVLM. Data represent as mean±SEM (n=5), *p<0.05, **p<0.01. |
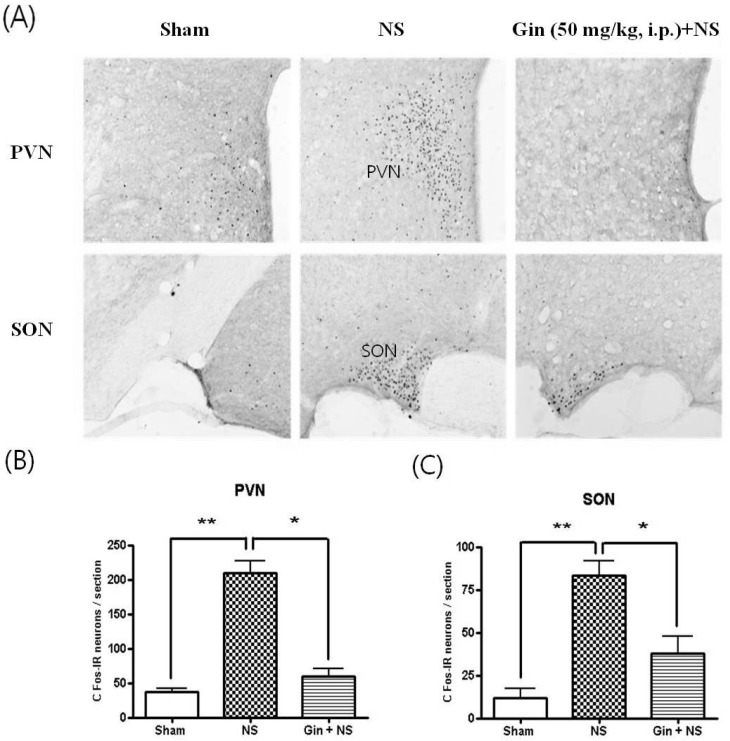 | Fig. 5Effects of ginsenosides on the number of Fos-IR neurons enhanced by NS to tooth in the hypothalamic paraventricular nucleus (PVN) and supraoptic nucleus (SON). (A) c-Fos immunoreactivity in PVN and SON. (B, C) Fos-IR neurons were significantly increased in the hypothalamic PVN and SON 1.5 h after NS to tooth and ginsenoside (Gin; 50 mg/kg, i.p.) reduced the number of Fos-IR neurons increased by NS to tooth. Data represent as mean±SEM (n=5), *p<0.05, **p<0.01. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download