Abstract
Mast cells are involved in allergic responses, protection against pathogens and autoimmune diseases. Dexamethasone (Dex) and other glucocorticoids suppress FcεRI-mediated release of inflammatory mediators from mast cells. The inhibition mechanisms were mainly investigated on the downstream signaling of Fc receptor activations. Here, we addressed the effects of Dex on Fc receptor expressions in rat mast cell line RBL-2H3. We measured mRNA levels of Fc receptors by real-time PCR. As expected, Dex decreased the mRNA levels of activating Fc receptor for IgE (FcεR) I and increased the mRNA levels of the inhibitory Fc receptor for IgG FcγRIIb. Interestingly, Dex stimulated transcriptions of other activating receptors such as Fc receptors for IgG (FcγR) I and FcγRIII. To investigate the mechanisms underlying transcriptional regulation, we employed a transcription inhibitor actinomycin D and a translation inhibitor cycloheximide. The inhibition of protein synthesis without Dex treatment enhanced FcγRI and FcγRIII mRNA levels potently, while FcεRI and FcγRIIb were minimally affected. Next, we examined expressions of the Fc receptors on cell surfaces by the flow cytometric method. Only FcγRIIb protein expression was significantly enhanced by Dex treatment, while FcγRI, FcγRIII and FcεRI expression levels were marginally changed. Our data showed, for the first time, that Dex regulates Fc receptor expressions resulting in augmentation of the inhibitory receptor FcγRIIb.
Mast cells are hematopoietic origin cells that take part in allergic reactions and autoimmune diseases [1-4]. Stimulation of mast cells elicits inflammatory responses, such as degranulation, production of lipid-mediated inflammatory mediators, and cytokine production. Granules of mast cells contain histamine, proteases, β-hexosaminidase and many inflammatory molecules.
Mast and other immune cells express receptors for Fc region of IgG (FcγR) and IgE (FcεR). The FcγR have two opposing families: activating Fc receptors (FcγRI, FcγRIIa and FcγRIII) and an inhibitory Fc receptor (FcγRIIb). FcεRI also is an activating receptor involved in allergic reactions. Balanced signaling between activating and inhibitory Fc receptors regulates immune system activity. An imbalance might result in the development of allergic and autoimmune diseases [5]. FcγRIIb is only one inhibitory receptor that suppresses many aspects of immune responses such as autoimmunity and infection [6]. Activating receptors are associated with a Src-family tyrosine kinase Lyn. They bind to corresponding antibodies and are cross-linked through multivalent antigens specific to the antibodies. This event activates Src family tyrosine kinases that phosphorylate tyrosine residues in immunoreceptor tyrosine-based activation motifs (ITAMs) which are in the cytoplasmic parts of receptors. Phosphorylated IATMs recruit signaling molecules that have Src homology (SH) 2 domain-containing proteins and then propagate activation signals [7]. The activation of FcεRI receptors stimulates Lyn, Fyn and subsequently Syk. Syk phosphorylates tyrosine residues in an adaptor molecule, linker for activation of T cells (LAT). Phosphorylated LAT recruits phospholipase C (PLC) and Grb2. PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate to produce diacylglycerols and inositol 1,4,5-trisphosphates. They result in the activation of protein kinase C (PKC) and calcium ion mobilization required for degranulation. Grb2 recruits Ras GTPase and activates extracellular receptor-activated kinase (ERK) that is involved with phospholipase A2 activation and cytokine gene expression. Fyn phosphorylates Gab2 and phosphorylated Gab2 recruits phosphatidylinositol 3-kinase (PI3-kinase) which is essential for the phosphorylation of the survival factor Akt by the phosphoinositide-dependent kinase (PDK). In contrast, the inhibitory receptor FcγRIIb has an immunoreceptor tyrosine-based inhibition motif (ITIM) that is phosphorylated by Src family kinases [6]. Phosphorylated ITIM recruits SH2 domain containing phosphatase (SHP) and phosphatidylinositol 5'-phosphatase (SHIP). They remove phosphate groups in activating signaling molecules and, thereby, downregulate activation signals. The balance between activating and inhibitory receptors maintains homeostasis in immune cells.
Glucocorticoids are extremely potent immune-suppressive agents that must be carefully used when treating immune diseases because of their undesirable side effects [8]. The molecular action mechanism of glucocorticoids is complex and still an important topic of study [9]. Glucocorticoids interact with intracellular glucocorticoid receptors (GR) and bind as dimers to glucocorticoid response elements (GRE), namely GGTACAAnnTGTYCTK and variants thereof on genes. This binding stimulates gene transcription, a process referred to as transactivation [10]. In contrast, glucocorticoids suppress cytokine gene transcriptions. They interact with GRs and then with transcription factors or co-activators to suppress cytokine gene transcriptions by a process referred to as transrepression. In addition to these negative or positive regulations of gene transcriptions, glucocorticoids regulate mRNA stabilities of target genes [11,12]. Furthermore, glucocorticoids exhibit rapid effects by nongenomic actions [13,14]. In mast cells, inhibitory mechanisms of glucocorticoids on FcεRI-mediated mast cell activation are mainly investigated in downstream signaling molecules of the receptor such as upregulating downstream of tyrosine kinase (Dok)-1 [15], DUSP1 [16,17] and Src-like adaptor protein (SLAP) [17,18].
Here, we addressed regulation of Fc receptors by a glucocorticoid Dex in rat RBL-2H3 mast cells. Using actinomycin D and cycloheximide as inhibitors for transcription or translation, respectively, we demonstrated that Dex increased mRNA levels of FcγRI, FcγRIIb and FcγRIII but decreased FcεRI transcript level. Although protein expressions of FcεRI, FcγRI and FcγRIII on cell surfaces were slightly changed, the expression of inhibitory receptor FcγRIIb increased significantly. Our data suggests that Dex suppresses Fc receptor-mediated mast cell activations by shifting Fc receptor expression toward the inhibitory receptor FcγRIIb on their cell surfaces.
RBL-2H3 cells were maintained in minimal essential medium (MEM) supplemented with 15% fetal calf serum, 2 mM glutamine, and an antibiotic-antimycotic solution. For transfection with siRNAs, cells were detached with trypsin-EDTA solution, and 2×106 cells were pelletted and suspended in 100 µl of Nucleofector Solution R (Amaxa). The suspension was mixed with siRNA against FcγRIIb (Invitrogen) at the concentration of 50 nM and transfected by electroporation program X-001 (Amaxa). The transfected cells were transferred to complete medium containing 100 ng/ml DNP-specific IgE and were cultured in 24 well plate (0.1×106 cells/1 ml/well).
RBL-2H3 cells were plated in 6-well (0.25×106 cells/2.5 ml/well) plates for measurement of mRNA. Cells were incubated for 24 h and treated with 100 nM Dex for the indicated time. Actinomycin D or/and cycloheximide were added before Dex treatment at 200 ng/ml or/and 1 µg/ml, respectively. They were washed twice with PBS and then lysed with 1 ml Easy blue (iNTRON). Total RNAs were purified from cells, and 1 µg of total RNAs was used for synthesis of cDNA in accordance to manufacturer's protocol (ELPiS). Transcript levels of β-actin (an internal control to calculate fold induction) and target genes were assayed by real-time PCR (Applied Biosystems) with following primers: β-actin, TCTGTGTGGATTGGTGGCTC TA, CTGCT TGCTGATCCACATCTG; FcεRI, GGACGACA TTGCTTTC AAGTACTC, TGGTAGCTGCCACTGTCATTAAA; FcγRI, CC TGGAGGACAGGAGCACAT, TCGCACCAGTA TGATCCA TCA; FcγRIIb, TCTGGCATCAAGCCCAAGCCA, CGCTGA TGCCGGTCTCCTCC; FcγRIII, CCACTAAGCG GCTGTT CCA, ACCTTAAATACAGGCGTGTTTCG. The fold changes in transcript levels were calculated using the 2-ΔΔCt method [ΔΔCt=(Cttarget gene-Ctβ-actin) Experimental groups-(Cttarget gene-Ctβ-actin)Control].
RBL-2H3 cells were detached from culture plates by repeated pipetting and then washed with Dulbecco's PBS containing 0.1% sodium azide (FACS buffer). For flow cytometric analysis, 1 µg of FITC-conjugated mouse IgE (BD Pharmingen), PE mouse anti-Mouse CD64 a and b alloantigens (BD Pharmingen), FITC mouse anti-rat CD32 (BD Pharmingen) or CD16 (ASH 1975) (Santa Cruz) was incubated with 1×106 cells in 100 µl FACS buffer for 1 h at 4℃. Alexa Fluor 488 chicken anti-mouse IgG (Life Technology) was used for detection of CD16. Cells were washed with FACS buffer and these receptor levels were measured by flow cytometry (Becton Dickinson, Canto II).
The degree of mast cell degranulation was assessed via measurement of granule enzyme β-hexosaminidase. RBL-2H3 cells were plated in 24-well (0.1×106 cells/1 ml/well). Cells were incubated for 24 h at 37℃ in culture medium containing 100 ng/ml anti-DNP-IgE and treated with 100 nM Dex for the indicated time. The cultures were washed twice with glucose saline/PIPES buffer (119 mM NaCl, 5 mM KCl, 5.6 mM glucose, 0.4 mM MgCl2, 25 mM PIPES pH 7.2, 1 mM CaCl2, 1% bovine serum albumin) and stimulated with 100 ng/ml DNP-HSA in saline/PIPES buffer for 20 min for assay of the β-hexosaminidase in medium and cells [19]. Data were expressed as a percent of cellular β-hexosaminidase that was released into the medium with the equation; % of release = stimulated release/(release+retained in the cells)×100.
To examine the inhibitory effects of Dex on FcεRI-mediated activation, we treated Dex to RBL-2H3 cells 6 h and 18 h before stimulation of FcεRI. We measured the extent of β-hexosaminidase release into the medium by degranulation process. Antigen stimulation produced 38% degranulation efficiency. Pretreatment of Dex for 6 h or 18 h inhibited 58% or 79% of degranulation, respectively (Fig. 1) in agreement with previous reports [18,20]. The inhibition mechanisms of glucocorticoids were described mainly on the regulation of downstream signaling molecules of Fc receptors [15-18]. Therefore, we addressed the regulation of Fc receptors by Dex.
Although anti-inflammatory effects of glucocorticoids have been attributed, in part, to upregulation of the expressions of inhibitory molecules of Fc receptor signaling, the effect of glucocorticoids on Fc receptor itself remains to be unstudied. To examine if Dex regulates Fc receptor expressions in mast cells, we assessed the kinetics of induction of Fc receptor mRNAs by Dex in RBL-2H3 cells. The real time RT-PCR analysis of RBL-2H3 cells indicated 50% decrease in mRNA levels for FcεRI 6 h after the addition of Dex. The amount of FcεRI mRNAs reached near minimal levels by 18 h and remained at decreased levels for at least 24 h (Fig. 2A). Next, the effects of Dex on mRNA levels of activating Fc γ receptors FcγRI and FcγRIII were investigated. Unexpectedly, transcript levels of these activating receptors increased in the presence of immune suppressive agent Dex. The amount of FcγRI mRNAs rapidly reached the highest level 3 h after the treatment of Dex by 17-fold, then declined relatively rapidly and reached basal levels (Fig. 2B). The mRNA level of FcγRIII increased gradually and showed maximal level 18 h after Dex treatment by 3-fold more (Fig. 2D). We then investigated transcript regulation of the inhibitory receptor FcγRIIb by Dex. The mRNA level gradually increased as expected and was maximal 18 h after treatment of Dex and remained constant for up to 24 h (Fig. 2C). The expression profiles of those Fc receptors indicated that Dex regulates their expressions differentially.
To examine mechanisms underlying regulation mechanisms of Fc receptor expressions by Dex, we investigated the effect of actinomycin D on Fc receptor expressions by Dex (Fig. 3). The inductions of FcγRI, FcγRIIb and Fcγ RIII transcripts by Dex were blocked by actinomycin D pretreatment. These results indicated that Dex induced transcriptions of these genes through the process of transactivation. In contrast, the amount of FcεRI transcripts was reduced by Dex. This means that transrepression mechanism of Dex inhibited the transcription of FcεRI gene. We employed a protein synthesis inhibitor cycloheximide to determine if proteins involved in mRNA stabilities regulate the transcript levels. Cycloheximide augmented FcγRI and FcγRIII mRNA levels by 5- and 8-fold, respectively (Fig. 3B and D). These results suggest that their mRNAs are constitutively produced without stimulation by Dex and that mRNA destabilizing proteins degrade their mRNAs. Meanwhile, Dex can stimulate their transcriptions (Fig. 2). Therefore, co-treatment of Dex and cycloheximide which results in the induction of transcriptions by Dex and reduction of mRNA destabilizing protein synthesis enhanced transcript levels maximally and in an additive manner. However, transcripts of FcεRI and FcγRIIb were not affected by cycloheximide treatment (Fig. 3A and C). These results suggest that mRNA levels of FcεRI and FcγRIIb are regulated mainly by transrepression and transactivation mechanisms of Dex, respectively, while mRNA levels of FcγRI and FcγRIII are controlled through the transactivation mechanism by Dex and mRNA destabilizing proteins.
The effects of Dex on production of Fc receptor transcripts raise the possibility that these genes are regulated, at least in part, through GRE. The MULAN program [21] was used to search for predicted GREs that are evolutionarily conserved in the Fc receptor genes of mouse and rat. Predicted GRE sites were present only in FcγRIIb gene. Two GREs were in reverse directions to the consensus GRE. One was upstream of the transcription start site and the other was within the fifth intron (Fig. 4A). The first putative GRE was CAGAACTGATTATGTA and the second was CAGAAC AGAGTTAAAA. These GREs are notable because of their location in the chromosome. The FcγRIIb gene is close to FcγRIII genes (Fig. 4B). Therefore, these GREs are candidate regulators for both genes since FcγRIII is also upregulated by Dex in RBL-2H3 cells (Fig. 2C).
Surface expressions of Fc receptors by Dex were examined by flow cytometric analysis. Dex treatment did not alter FcεRI protein levels until 18 h (Fig. 5A), although Dex decreased its transcripts (Fig. 2A). While mRNA levels of FcγRI and FcγRIII increased significantly in the presence of Dex (Fig. 2B and D), changes of their surface expressions were rather marginal (Fig. 5B and D). In contrast, FcγRIIb surface levels significantly increased 1.8-fold by Dex (Fig. 5C) pertaining to its transcript regulation (Fig. 2C). The augmentation of Fc receptor transcripts did not always result in their protein expression. These data suggest that Dex stimulates expression of an inhibitory Fc receptor FcγRIIb on the cell surface without increases in protein expressions of activating receptors FcεRI, FcγRI and FcγRIII.
Transfection of siRNA against FcγRIIb mRNA decreased its transcript level and attenuated Dex-mediated enhancement of FcγRIIb transcripts by 50% (Fig. 6A). Accordingly, cell surface expression of FcγRIIb was reduced in the presence of Dex or in the absence of Dex by 50% (Fig. 6A). We measured degranulation efficiency by cross-linking of FcεRI in these conditions. Interestingly, downregulation did not affect degranulation efficiency (Fig. 6B).
We showed that a glucocorticoid Dex regulated Fc receptor transcriptions and enhanced the protein expression of an inhibitory receptor, FcγRIIb, on cell surfaces of RBL-2H3 mast cells. Glucocorticoids regulate transcription directly or indirectly. Ligand-bound glucocorticoid receptors interact with AP1 and NF-κB which are transcription factors that promote inflammatory cytokine production [10]. These interactions inhibit transcriptional activities of AP-1 or NF-κB and result in the suppression of inflammatory cytokine production. Dex repressed FcεRI transcription and enhanced transcriptions of IgG receptors FcγRI, Fcγ RIIb and FcγRIII (Fig. 2). We assumed that the trans-repression mechanism may be involved in regulating FcεRI gene expression. Although the transcript level of FcεRI decreased by Dex, cell surface expression of FcεRI protein did not change (Fig. 5A). Longer exposure to Dex would decrease FcεRI proteins level. In mouse mast cells, Dex downregulated cell surface expression of FcεRI [20,22] in the same exposure time to Dex with our experimental conditions. FcεRI proteins of a rat mast cell RBL-2H3 may be more stable than proteins of mouse origin cells.
Unexpectedly, an immune suppressive agent, Dex, stimulated transcriptions of the activating receptors FcγRI and FcγRIII (Fig. 2B and D). The inhibition of protein synthesis alone strongly increased mRNA levels as much as Dex did and augmented the Dex-induced mRNA levels of FcγRI and FcγRIII (Fig. 3). Proteins involved in mRNA degradation, such as tristetraproline [23], may degrade FcγRI and FcγRIII transcripts. Their mRNAs may be constitutively produced and degraded by mRNA-destabilizing proteins that are expressed in a Dex-independent manner. Although enhancement of activating receptors by the immune suppressive agent, Dex, seems to be absurd, this can be explained by the fact that the expression ratio of activating and inhibitory Ig receptors determines the outcome of immune responses [6]. The experiment performed with monocytes from immune thrombocytopenia patients showed that Dex induces both activating and inhibitory receptors, with FcγRIIb at higher amplitudes. Also, FcγRIII expression in human neutrophils is sometimes increased slightly by Dex [24]. Accordingly, our data suggest that Dex shifted mast cell Fc receptor balance toward inhibitory FcγRIIb (Fig. 5). Alternatively, increased Fc receptors having ITAM motifs by Dex may act as negative immune regulators. Recent studies showed that ITAMs of activating receptors recruit negative signaling molecules such as SHP-1 in some conditions and then inhibit other activating receptor signals [25,26]. Dex treatment might make favorable cell conditions for ITAM of FcγRI to act an inhibitory ITAM.
Dex treatment suppressed FcεRI-mediated degranulation efficiency (Fig. 1). However, reduction of FcεRI protein expression on cell surfaces was not observed (Fig. 5). The shifting Fc receptor expression toward inhibitory receptor FcγRIIb by Dex would contribute to the reduction of degranulation efficiency in addition to induction of inhibitory molecules in the downstream signaling of FceRI activation [17,18]. However, downregulation of FcγRIIb expression by transfection with FcγRIIb siRNA did not show any significant effect on degranulation efficiency (Fig. 6A). These data suggest that binding affinity of IgE to Fcγ RIIb in vitro may not be enough to inhibit FcεRI-mediated activation signals even though the IgE-mediated degranulation is augmented in FcγRIIb-deficient mice in vivo [27]. Another possibility is that 50% reduction of FcγRIIb expression by the siRNA transfection (Fig. 6) was not enough to inhibit FcεRI-mediated degranulation. The complete downregulation of FcγRIIb transcripts in FcγRIIb-deficient mice may contribute to the decrease in degranulation efficiency.
FcγRI, FcγRIII and FcγRIIb genes are clustered at chromosome 13 in rats and chromosome 1 in mice (Fig. 4). In humans, FcγRIII and FcγRIIb are also close. The predicted transcription factor binding sites in evolutionarily conserved regions between species give more valuable idea. We found two evolutionary conserved GREs in FcγRIIb gene region between a rat and a mouse, but not between human and murine. These conserved elements may regulate murine FcγRI, FcγRIIb and FcγRIII genes coordinately in respose to Dex as shown in this investigation (Fig. 2B and D). These receptor expression mechanisms in response to Dex would be different from human and murine.
FcγRIIb is the only inhibitory Fc receptor that is expressed on mast cells and other immune cells such as granulocytes, myeloid cells and lymphoid cells, missing only from T and NK cells. It acts as a key negative regulator to maintain immune balance from activating Fc receptor signals. Mast cells express IgE receptors and also IgG receptors (FcγRI, FcγRIIb, FcγRIII). Mast cells elicit IgG-mediated immune responses supported by the observation that IgG cross-linking of mast cells, basophils, and macrophages resulted in Fyn- and Lyn-regulated mediator release in vitro [28]. Up-regulation of FcγRIIb by Dex in mast cells may contribute to Dex effects as an immune suppressive agent in the Arthus reaction where FcγRIII on mast cells is necessary for this inflammatory response [29]. Recent reports suggest that mast cells are involved in the developments of immune diseases such as multiple sclerosis, inflammatory arthritis, atherosclerosis and diet-induced obesity and diabetes [1-4] where autoimmunity is possibly involved. FcγRIIb deficiency makes normally resistant mouse strains to be susceptible to several antibody-or immune complex-dependent models of autoimmunity [6]. Our finding that Dex enhanced FcγRIIb expression on mast cells may give a rationale for use of Dex in the treatment of autoimmune diseases where mast cells are involved.
Figures and Tables
 | Fig. 1Inhibition of degranulation by Dex in RBL-2H3 cells. RBL-2H3 cells were cultured for 18 h with IgE (at 100 ng/ml) and Dex (100 nM) was treated for indicated times. After sensitization with IgE specific to DNP overnight, cells were washed and then stimulated with antigens (DNP-HSA) for 15 min. Data are expressed as mean±SD, n=3. **p<0.01 versus no antigen treatment. |
 | Fig. 2mRNA levels of FcεRI and FcγRs by Dex. Transcript levels of FcεRI (A), FcγRI (B), FcγRIII (C) and FcγRIIb (D) were measured following treatment with 100 nM Dex to RBL-2H3 cells for the indicated time. Real time PCRs were performed with cDNAs made from reverse transcriptase reaction from RNA extracts. Results are expressed as fold change relative to control. Filled squares are Dex treatment and filled circles are control. Data are expressed as mean±SD, n=3. **p<0.01 versus no Dex treatment. |
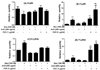 | Fig. 3Effects of actinomycin D and cycloheximide on Fc receptor transcript levels. Actinomycin D (AcD, 200 ng/ml) or cycloheximide (ChX, 1 µg/ml) was added 20 min before treatment of 100 nM Dex and transcript levels were determined 6 h thereafter. Expression analysis was performed by real-time PCR. Data are expressed as mean±SD, n=3. **p<0.01 versus no treatment. |
 | Fig. 4Organization of FcγRIIb genes. (A) Exons and introns are indicated by the numbered boxes and solid lines, respectively. Dashed lines indicate non-transcribed regions. (B) Genes surrounding the FcγRIIb gene. |
 | Fig. 5Effects of Dex treatment on cell surface expressions of Fcε RI, FcγRI, FcγRIIb and FcγRIII. Cultured RBL-2H3 cells were treated with Dex (100 nM) for 18 h, and cells were stained with antibodies specific to FcεR (A), FcγRI (B), FcγRIIb (C) and Fcγ RIII (D). The surface expressions of Fc receptors were verified by cell fluorescence analysis. Dex treatment or control is represented with filled or empty area, respectively. Data is a representative from three separate experiments. |
 | Fig. 6Effects of downregulation of FcγRIIb by transfection of anti-FcγRIIb siRNA. The effects of transfection with siRNA against FcγRIIb on its transcript level (A) and degranulation efficiency by the cross-linking of FcεRI (B). Cells were incubated 24 h after transfection with the siRNA. After cells were incubated for 18 h in the presence or absence of 100 nM Dex, degranulation efficiency was determined by β-hexosaminidase assay, and cell surface expression was evaluated by flow cytometric analysis. Data are expressed as mean±SD, n=3. Flow cytometric data is a representative from three separate experiments. |
ACKNOWLEDGEMENTS
We are grateful to Dana Toy for writing this manuscript. This study was financially supported by research fund of Chungnam National University in 2009 and Chungnam National University Hospital Research Fund, 2011.
References
1. Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000. 191:813–822.
2. Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002. 297:1689–1692.
3. Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007. 13:719–724.
4. Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009. 15:940–945.
5. Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002. 2:580–592.
6. Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010. 10:328–343.
7. Scharenberg AM, Lin S, Cuenod B, Yamamura H, Kinet JP. Reconstitution of interactions between tyrosine kinases and the high affinity IgE receptor which are controlled by receptor clustering. EMBO J. 1995. 14:3385–3394.
8. Rosen J, Miner JN. The search for safer glucocorticoid receptor ligands. Endocr Rev. 2005. 26:452–464.
9. Clark AR. Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol. 2007. 275:79–97.
10. Ito K, Chung KF, Adcock IM. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2006. 117:522–543.
11. Smoak K, Cidlowski JA. Glucocorticoids regulate tristetraprolin synthesis and posttranscriptionally regulate tumor necrosis factor alpha inflammatory signaling. Mol Cell Biol. 2006. 26:9126–9135.
12. Ing NH. Steroid hormones regulate gene expression posttranscriptionally by altering the stabilities of messenger RNAs. Biol Reprod. 2005. 72:1290–1296.
13. Stahn C, Löwenberg M, Hommes DW, Buttgereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007. 275:71–78.
14. Stellato C. Post-transcriptional and nongenomic effects of glucocorticoids. Proc Am Thorac Soc. 2004. 1:255–263.
15. Hiragun T, Peng Z, Beaven MA. Dexamethasone up-regulates the inhibitory adaptor protein Dok-1 and suppresses downstream activation of the mitogen-activated protein kinase pathway in antigen-stimulated RBL-2H3 mast cells. Mol Pharmacol. 2005. 67:598–603.
16. Kassel O, Sancono A, Krätzschmar J, Kreft B, Stassen M, Cato AC. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001. 20:7108–7116.
17. Park SK, Beaven MA. Mechanism of upregulation of the inhibitory regulator, src-like adaptor protein (SLAP), by glucocorticoids in mast cells. Mol Immunol. 2009. 46:492–497.
18. Hiragun T, Peng Z, Beaven MA. Cutting edge: dexamethasone negatively regulates Syk in mast cells by up-regulating SRC-like adaptor protein. J Immunol. 2006. 177:2047–2050.
19. Ozawa K, Szallasi Z, Kazanietz MG, Blumberg PM, Mischak H, Mushinski JF, Beaven MA. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J Biol Chem. 1993. 268:1749–1749.
20. Benhamou M, Ninio E, Salem P, Hieblot C, Bessou G, Pitton C, Liu FT, Mencia-Huerta JM. Decrease in IgE Fc receptor expression on mouse bone marrow-derived mast cells and inhibition of paf-acether formation and of beta-hexosaminidase release by dexamethasone. J Immunol. 1986. 136:1385–1392.
21. Ovcharenko I, Nobrega MA, Loots GG, Stubbs L. ECR Browser: a tool for visualizing and accessing data from comparisons of multiple vertebrate genomes. Nucleic Acids Res. 2004. 32:W280–W286.
22. Yamaguchi M, Hirai K, Komiya A, Miyamasu M, Furumoto Y, Teshima R, Ohta K, Morita Y, Galli SJ, Ra C, Yamamoto K. Regulation of mouse mast cell surface Fc epsilon RI expression by dexamethasone. Int Immunol. 2001. 13:843–851.
23. Ishmael FT, Fang X, Galdiero MR, Atasoy U, Rigby WF, Gorospe M, Cheadle C, Stellato C. Role of the RNA-binding protein tristetraprolin in glucocorticoid-mediated gene regulation. J Immunol. 2008. 180:8342–8353.
24. Petroni KC, Shen L, Guyre PM. Modulation of human polymorphonuclear leukocyte IgG Fc receptors and Fc receptor-mediated functions by IFN-gamma and glucocorticoids. J Immunol. 1988. 140:3467–3472.
25. Pfirsch-Maisonnas S, Aloulou M, Xu T, Claver J, Kanamaru Y, Tiwari M, Launay P, Monteiro RC, Blank U. Inhibitory ITAM signaling traps activating receptors with the phosphatase SHP-1 to form polarized "inhibisome" clusters. Sci Signal. 2011. 4:ra24.
26. Kanamaru Y, Pfirsch S, Aloulou M, Vrtovsnik F, Essig M, Loirat C, Deschênes G, Guérin-Marchand C, Blank U, Monteiro RC. Inhibitory ITAM signaling by Fc alpha RI-FcR gamma chain controls multiple activating responses and prevents renal inflammation. J Immunol. 2008. 180:2669–2678.
27. Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, Ravetch JV, Takai T. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999. 189:1573–1579.
28. Falanga YT, Chaimowitz NS, Charles N, Finkelman FD, Pullen NA, Barbour S, Dholaria K, Faber T, Kolawole M, Huang B, Odom S, Rivera J, Carlyon J, Conrad DH, Spiegel S, Oskeritzian CA, Ryan JJ. Lyn but not Fyn kinase controls IgG-mediated systemic anaphylaxis. J Immunol. 2012. 188:4360–4368.
29. Sylvestre DL, Ravetch JV. A dominant role for mast cell Fc receptors in the Arthus reaction. Immunity. 1996. 5:387–390.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download