Abstract
Anti-tumor activity of the proteins from Gecko (GP) on cervical cancer cells, and its signaling mechanisms were assessed by viable cell counting, propidium iodide (PI) staining, and Western blot analysis. GP induced the cell death of HeLa cells in a dose-dependent manner while it did not affect the viability of normal cells. Western blot analysis showed that GP decreased the activation of Akt, and co-administration of GP and Akt inhibitors synergistically exerted anti-tumor activities on HeLa cells, suggesting the involvement of PI3-kinase/Akt pathway in GP-induced cell death of the cancer cells. Indeed, the cytotoxic effect of GP against HeLa cells was inhibited by overexpression of constituvely active form of Akt in HeLa cells. The candidates of the functional proteins in GP were analyzed by Mass-spectrum. Taken together, our results suggest that GP elicits anti-tumor activity against HeLa cells by inhibition of PI3-kinase/Akt pathway.
Cervical cancer is the third most common cancer among females worldwide, and around 275,000 women die of the cancer every year [1]. Although it can be effectively cured by simple surgery when it is found at the early stage, prolonged and profound chemotherapy or radiotherapy are required in more advanced stages or metastatic stage, which may accompany a variety of side effects. Furthermore, drug-resistance or toxicity of synthetic agents remains to be an obstacle to an effective treatment. Thus, a novel therapeutic strategy with more safety and less toxicity is required for highly effective cure of the cervical cancer.
Gecko, a genus of lizards, has been traditionally used as an Oriental medicine in the form of pill, powder and mastic for a variety of inflammatory diseases such as tuberculosis and osteomyelitis and syrinx [2]. Furthermore, it has been reported that Gecko has an anti-tumor effect on several cancers including gastric cancer, liver cancer and esophageal carcinoma [3-5]. However, due to the uncertain scientific backgrounds of these therapeutic effects, the Gecko still may not gain global reliability as an anti-cancer drug, although several attempts have been made to develop new anti-cancer pharmaceuticals from Chinese herbal medicine [6-8].
The major goal of this study was to investigate whether Gecko also has an anti-tumor activity on non-digestive tissue cancer such as cervical cancer using HeLa cells, and to elucidate the signaling mechanisms of anti-tumor action of the Gecko. As a result, we found that the proteins from Gecko (GP) were able to selectively eliminate HeLa cells, while it did not affect viability of normal cells. The GP inhibited Akt activation, and the overexpressing constituvely active form of Akt rescued the GP-induced cell death of HeLa, suggesting that the GP induces the specific cell death of the cancer cells via inhibition of PI3-kinase pathway.
All cells were purchased from the American Type Culture Collection (ATCC). Cells were cultured in DMEM (HyClone) supplemmented with 10% fetal bovine serum (FBS; HyClone) and penicillin/streptomycin (100 U/ml; HyClone) at 37℃ in a humidified incubator with 5% CO2.
Young (4~6 weeks) Eublepharis. Macularius were obtained from a commercial supplier (Mowglipet, Seoul, Korea), and captive bred. Briefly, the Geckos were housed individually in standard mouse-sized polycarbonate enclosures in an isolated room with an ambient humidity of 40~50% at room temperature of ~24℃. Animals were fed daily a diet of gut-loaded mealworms (larval Tenebrio spp.) dusted with powdered calcium and vitamin D3 (cholecalciferol) supplement.
Animals of 8 to 11 cm in length were anaesthetized in 0.02% to 0.05% MS-222 (Argent Chemical Laboratories, Redmond, WA, USA) and tails were amputated with a size of 0.5 cm. The amputated tails were rinsed in sterile phosphate buffered saline (PBS) and homogenized by using a homogenizer. The homogenates were centrifuged (13,000 rpm for 10 min at 4℃) and the supernatants were passed through a 0.45 µm of syringe filter.
All cells (5×104/ml cell suspension) were seeded on to 24-well plates at 5×104/ml in DMEM medium with 10% FBS. Cells were treated with designated concentrations of GP and further incubated for 48 hours. Then, the cells were trypsinized (10× trypsin-EDTA, Gibco) and the viable cell numbers were counted using a hematocytometer under optical microscope.
HeLa cells (1×106) were seeded into a 6-well plate and cultured for overnight. Then, the cells were transfected with 2 µg of constituvely active form of myristoylated Akt expression vector (Myr-Akt) or empty vector (pUSEamp, Upstate Technology) using LipofectAMINE according to the manufacturer's procedure. After transfection, cells were cultured in 10% fetal bovine serum-supplemented DMEM for 24 hours, then subjected to 0.1% DMSO or GP treatment for 48 h. These cells were then used for PI staining, cell counting, and Western blot analysis.
Cells were lysed in lysis buffer [20 mM Tris-HCl (pH 6.8), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% TritonX-100] containing a protease inhibitor (complete-Mini, Roche) for 20 minutes on ice, and then centrifugated at 13,000 g for 20 minutes at 4℃. Twenty mg of the proteins were resolved on 12% sodium dodecyl sulfate-polyacrylamide gel and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were incubated sequentially with primary antibodies and HRP-conjugated secondary antibodies. Immunoreactivity was detected with Enhanced peroxidase detection (EPD, ELPIS Biotec. INC) on X-ray film (Sigma-Aldrich).
200~250 µg protein was loaded onto a 11 cm 4~7 linear IPG strip for separation in the first dimension, and the second dimension separation was on a standard 12% SDS-PAGE gel. The gels were visualized with Silver staining according to the manufacturer's instructions. Spots were identified and analyzed using the PDQuest v8.0 software (Biorad). Background subtraction and normalization were automatically carried out by the software programs.
The separated proteins in SDS-PAGE gels were visualized by silver staining. The stained gel images were compared with the original DeCyder analysis experiments and matched. The spots of interest were either manually excised or automatically detected and excised using the Xcise™ apparatus (Shimadzu Biotech, Japan). Gel pieces were washed twice with 150 µl of 100 mM ammonium bicarbonate (pH 8.2) and 70% v/v acetylnitrile (ACN), and dried at 37℃ for 20 min. Trypsin in 50 mM ammonium bicarbonate (20 µg/µl) was added to each gel piece and incubated at 37℃ for 2 h. Peptides were then extracted using a mixture containing 20 µl of 0.1% v/v trifluoroacetic acid (TFA) and 70% ACN. The peptide solution was either manually or automatically desalted and concentrated using ZipTips™ (Millipore, Bedford, MA) (8,12), and spotted onto an Axima Maldi target plate. The peptide mass spectra of tryptic peptides were generated by using an Axima CFR+ matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF-MS; Shimadzu Biotech, Japan). The peptide masses were matched with the theoretical peptide masses obtained from the mouse database of the NCBInr using Mascot with the automated Mascot Daemon v.2.0 (Matrix Sciences, London, UK).
Gecko has been previously reported to have anti-tumor effect on several cancers in digestive system such as gastric cancer, liver cancer and esophageal carcinoma. In this study, we investigated whether proteins from Gecko (GP) also have the same effect on cervical cancer using HeLa cell. Microscopic observations showed that administration of GP decreased number of the HeLa cells in a dose dependent manner (Fig. 1A), and the cell death by GP was confirmed by propidium iodide (PI) staining in which GP treatment increased number of the PI-positive cells (Fig. 1B). Cell counting assay also showed that GP decreased viable cell numbers of HeLa cells while heat-inactivated proteins did not affect the viability of the cancer cells (Fig. 2). In addition, GP did not affect the survival of normal cells such as human embryonic kidney cells (HEK293), mouse fibroblast cells (NIH3T3), and mouse myoblast cells (C2C12). Taken together, these results suggest that GP has cytotoxic effect specifically on cancer cells without affecting normal cells.
PI3-kinase/Akt is the typical enzymes that regulate cellular growth, proliferation and survival. Thus, we investigated whether PI3-kinase/Akt are involved in GP-induced cell death of HeLa cells. Western blot analysis showed that GP decreased the level of Akt activation in a dose-dependent manner (Fig. 3A). In addition, co-administration of GP and PI3-kinase inhibitors synergistically decreased the viable cell numbers of HeLa (Fig. 3B), suggesting that GP inhibits the PI 3-kinase pathway, thereby decreasing survival of HeLa cells.
In order to confirm the involvement of PI3-kinase/Akt in GP-induced cell death of HeLa cells, we performed cytotoxic assay with the HeLa cells which overexpress constituvely active form of Akt (Fig. 4A). As assessed by PI staining, addition of GP did not change the viability of HeLa cells when active form of Akt is overexpressed while it increased cell death of control cells (Fig. 4B). These results prove that GP induces cell death of HeLa cells via inhibition of PI3-kinase/Akt pathways.
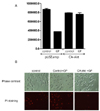
To identify the major proteins that may possess the functional anti-tumor activities in GP, we next performed 2-dimensional (2D) electrophoresis with the total proteins from Gecko (Fig. 5A). Among the 292 spots from 2D gel, we selected 18 major spots, and they are subjected to mass-spectrometry. As in the Table 1 and Fig. 5B, they are mostly involved in cellular metabolism, protease inhibitors, and cellular signalling.
Previously, Gecko has been reported to have therapeutic effects against various inflammatory diseases [9]. Furthermore, Gecko also has been used as an Oriental medicine for cancers in digestive system, including gastric cancer, liver cancer and esophageal carcinoma [3-5]. In addition, recent report showed that the sulfated polysaccharide from Gecko inhibited proliferation and migration of human hepatoma cell line [10-12].
However, the absence of the scientific backgrounds of the mechanisms remains as an obstacle for Gecko to gain global interests as an anti-cancer drug. Moreover, most studies about pharmaceutical effects of Gecko have been performed with the whole dried mixtures or the mastic of the animal until recently. Here, we investigated the therapeutic effects of Gecko proteins against the cervical cancer cells, and elucidated its critical mechanisms in anti-tumor action. The sole anti-tumor effect of proteins itself from Gecko was proven by the fact that the heat-inactivated proteins did not have any effects on the proliferation or the survival of the cancer cells. Moreover, our observations, in which GP did not show any toxicity against the normal cells, confirmed the specificity of GP as an effective anti-tumor drug.
Previous study with the whole dry argued that the mechanism of anti-tumor action of Gecko involves decrease of VEGF and bFGF protein expression in tumor tissues [2]. Although VEFG and bFGF are critical for tumor growth, they may not be the directly related to the cell death or the survival of the cancer cells. Thus, we investigated the involvement of PI3-kinase/Akt pathway in anti-tumor activities of the GP. In fact, PI3-kinase/Akt has been well known as a critical factor for tumor formation, metastasis, and tumor regression [13-15]. Furthermore, VEGF and PI3-kinase are often closely related to each other in regulating certain cellular physiologies such as lymphatic metastasis [16]. Indeed, inhibitors of PI3-kinase/Akt pathway have been tried as anti-cancer pharmaceuticals [17,18]. Our results also showed that the GP decreased the activation of Akt, and the co-administration of inhibitors of PI3-kinase with the GP synergistically decreased the survival of the cervical cancer cells, as expected. Moreover, the cell death by the GP was recovered by overexpression of constituvely active form of Akt in the HeLa cells. The proteomic analysis in this study revealed that the major proteins contained in GP are involved in cellular metabolism, material transport, signaling, cytoskeleton rearrangement, and protease inhibition. Indeed, all of these events are well-known to be involved in cell survival, proliferation and apoptosis. Especially, it has recently been reported that the protease inhibitors or inhibition of the proteases are critical for anti-tumor efficacy of the drugs against certain cancers such as the colon cancer [19,20] or liver cancer [21]. Thus, tryptase-inhibitor in GP, which were identified in this study may be a good candidate as a safe and efficient anti-cancer drug against the cervical cancer although the further functional study may be required.
In conclusion, this study has demonstrated that the proteins from Gecko have the anti-tumor activity on the cervical cancers, possibly by the downregulation of PI3-kinase/Akt pathway. Further functional studies such as identification of functional peptides in GP may greatly contribute to the efficient drug development of a variety of cancers such as cervical, colon, and liver cancers.
Figures and Tables
 | Fig. 1Effect of GP on survival of HeLa cells. HeLa cells were cultured with designated concentrations of GP for 48 hours. Viable cells were observed with phase contrast microscope (A), and dead or dying cells were analyzed by PI staining (B). Data are representatives of three independent experiments. |
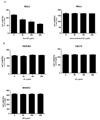 | Fig. 2Specific anti-tumor effect of GP against HeLa cells. (A) HeLa cell were treated with raw GP (left panel) or heat-inactivated GP (right panel) for 48 hours, and the viable cell numbers were counted. (B) Various types of normal cells were treated with raw GP for 48 hours and viable cell numbers were counted (data are averages of three independent experiments). |
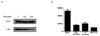 | Fig. 3Activation of MAP kinases. (A) HeLa cells were treated with designated concentration (0, 100, 200 µg/ml) of GP for 30 minutes, and the activation of Akt was analyzed by Western blot analysis. (B) HeLa cells were treated with wortmannin (Wort) and/or GP for 48 hours, and the viable cell numbers were counted. DMSO was included as a vehicle control. |
 | Fig. 4Confirmation of involvement of PI3-kinase/Akt in GP induced cell death of HeLa cells. (A) Control HeLa cells (pUSEamp) and the active Akt-overexpressing HeLa cells (CA-Akt) were treated with or without GP (200 µg/ml) for 48 hours, and the viable cell numbers were counted. Data are averages of three independent experiments. (B) After treatment of GP (200 µg/ml), the cells were stained with PI and the viability of the cells was investigated by microscopic analysis. Data are representative of three independent experiments. |
 | Fig. 5Protein separations of total GP on 2D gels, and categorization of the identified proteins by mass spectrometry. (A) After isoelectric focusing in pH gradient of the electric field, the electrophoretic separation is used in polyacrylamide gel (SDS-PAGE). Proteins were visualized after silver staining. (B) Identified proteins in Table 1 were categorized according to their cellular functions. |
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010. 127:2893–2917.
2. Liu F, Wang JG, Wang SY, Li Y, Wu YP, Xi SM. Antitumor effect and mechanism of Gecko on human esophageal carcinoma cell lines in vitro and xenografted sarcoma 180 in Kunming mice. World J Gastroenterol. 2008. 14:3990–3996.
3. Yang JX, Wang XM. Progress study and research on treating tumor of Gecko. Shijie Huaren Xiaohua Zazhi. 2006. 14:2428–2431.
4. Wu BD. Treatment of 105 cases of esophagus tumor by compound recipe of Gecko. Zhongguo Zhongxiyi Jiehe Zazhi. 1999. 19:502.
5. Song P, Wang XM, Xie S. Experimental study on mechanisms of lyophilized powder of fresh gekko Chinenis in inhibiting H22 hepatocarcinoma angiogenesis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006. 26:58–62.
6. Wang SJ, Zhang XY, Lu LB. Research and application of the pharmacological testing method using serum with Chinese herbal medicine. Zhongguo Shouyao Zazhi. 2004. 38:35–37.
7. Xue J, Xie ML. Advances in studies on methodology in serum pharmacology of Chinese materia medica. Zhongcaoyao. 2003. 34:9–11.
8. Iwama H, Amagaya S, Ogihara Y. Effect of shosaikoto, a Japanese and Chinese traditional herbal medicinal mixture, on the mitogenic activity of lipopolysaccharide: a new pharmacological testing method. J Ethnopharmacol. 1987. 21:45–53.
9. Ferreira FS, Brito SV, Saraiva RA, Araruna MK, Menezes IR, Costa JG, Coutinho HD, Almeida WO, Alves RR. Topical anti-inflammatory activity of body fat from the lizard Tupinambis merianae. J Ethnopharmacol. 2010. 130:514–520.
10. Chen D, Zhang X, Du Y, Jia B, Ka W, Sun D, Yao W, Wen Z. Effects of Gekko sulfated polysaccharide-protein complex on the defective biorheological characters of dendritic cells under tumor microenvironment. Cell Biochem Biophys. 2012. 62:193–201.
11. Wu XZ, Chen D, Han XQ. Anti-migration effects of Gekko sulfated glycopeptide on human hepatoma SMMC-7721 cells. Molecules. 2011. 16:4958–4970.
12. Chen D, Yao WJ, Zhang XL, Han XQ, Qu XY, Ka WB, Sun DG, Wu XZ, Wen ZY. Effects of Gekko sulfated polysaccharide-protein complex on human hepatoma SMMC-7721 cells: inhibition of proliferation and migration. J Ethnopharmacol. 2010. 127:702–708.
13. Zhu YF, Yu BH, Li DL, Ke HL, Guo XZ, Xiao XY. PI3K expression and PIK3CA mutations are related to colorectal cancer metastases. World J Gastroenterol. 2012. 18:3745–3751.
14. Hussain AR, Ahmed SO, Ahmed M, Khan OS, Al Abdulmohsen S, Platanias LC, Al-Kuraya KS, Uddin S. Cross-talk between NFkB and the PI3-kinase/AKT pathway can be targeted in primary effusion lymphoma (PEL) cell lines for efficient apoptosis. PLoS One. 2012. 7:e39945.
15. Qian C, Lai CJ, Bao R, Wang DG, Wang J, Xu GX, Atoyan R, Qu H, Yin L, Samson M, Zifcak B, Ma AW, Dellarocca S, Borek M, Zhai HX, Cai X, Voi M. Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling. Clin Cancer Res. 2012. 18:4104–4113.
16. Coso S, Zeng Y, Opeskin K, Williams ED. Vascular endothelial growth factor receptor-3 directly interacts with phosphatidylinositol 3-kinase to regulate lymphangiogenesis. PLoS One. 2012. 7:e39558.
17. Hong DS, Bowles DW, Falchook GS, Messersmith WA, George GC, O'Bryant CL, Vo AC, Klucher K, Herbst RS, Eckhardt SG, Peterson S, Hausman DF, Kurzrock R, Jimeno A. A Multicenter Phase I Trial of PX-866, an Oral Irreversible Phosphatidylinositol 3-Kinase Inhibitor, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2012. 18:4173–4182.
18. Le TK, Jeong JJ, Kim DH. Clionosterol and ethyl cholestan-22-enol isolated from the rhizome of Polygala tenuifolia inhibit phosphatidylinositol 3-kinase/Akt pathway. Biol Pharm Bull. 2012. 35:1379–1383.
19. Clemente A, Carmen Marín-Manzano M, Jiménez E, Carmen Arques M, Domoney C. The anti-proliferative effect of TI1B, a major Bowman-Birk isoinhibitor from pea (Pisum sativum L.), on HT29 colon cancer cells is mediated through protease inhibition. Br J Nutr. 2012. 108:suppl 1. S135–S144.
20. Brandi G, Tavolari S, De Rosa F, Di Girolamo S, Agostini V, Barbera MA, Frega G, Biasco G. Antitumoral efficacy of the protease inhibitor gabexate mesilate in colon cancer cells harbouring KRAS, BRAF and PIK3CA mutations. PLoS One. 2012. 7:e41347.
21. Sun L, Niu L, Zhu X, Hao J, Wang P, Wang H. Antitumour effects of a protease inhibitor, nelfinavir, in hepatocellular carcinoma cancer cells. J Chemother. 2012. 24:161–166.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download